8.52089
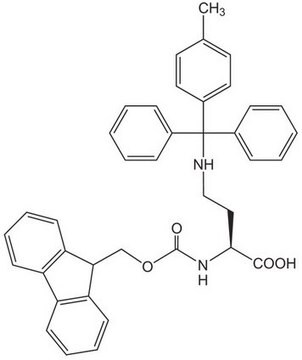
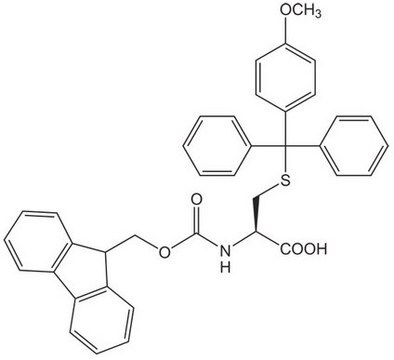
Fmoc-Dpr(Mtt)-OH
Novabiochem®
Synonym(s):
Fmoc-Dpr(Mtt)-OH, N-α-Fmoc-N-β-4-methyltrityl-L-diaminopropionic acid
About This Item
Recommended Products
Quality Level
product line
Novabiochem®
Assay
≥95% (TLC)
≥95.0% (HPLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
amine
storage temp.
15-25°C
InChI
1S/C38H34N2O4/c1-26-20-22-29(23-21-26)38(27-12-4-2-5-13-27,28-14-6-3-7-15-28)39-24-35(36(41)42)40-37(43)44-25-34-32-18-10-8-16-30(32)31-17-9-11-19-33(31)34/h2-23,34-35,39H,24-25H2,1H3,(H,40,43)(H,41,42)/t35-/m0/s1
InChI key
WDZDBCVDBMWMAM-DHUJRADRSA-N
General description
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] K. Barlos, et al., C. H. Schneider & A. N. Eberle (Eds), ESCOM, Leiden, 1993, pp. 283.
[2] A. Aletras, et al. (1995) Int. J. Peptide Protein Res., 45, 488.
[3] L. Bourel, et al. (2000) J. Peptide Sci., 6, 264.
[4] K. Barlos, personal communication.
[5] P. Hoogerhout, et al. (1999) J. Peptide Res., 54, 436.
[6] C. Park & K. Burgess (2001) J. Comb. Chem., 3, 257.
Linkage
Analysis Note
Appearance of substance (visual): powder
Identity (IR): passes test
Optical rotation α 25/D (c=1 in methanol): +10.0 - +13.0 °
Purity (TLC(157A)): ≥ 95 %
Assay (HPLC, area%): ≥ 95.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Novabiochem® product range has one of the largest collections of orthogonally and quasi-orthogonally protected tri-functional amino acids. These derivatives are useful tools for the synthesis of cyclic and branched peptides and peptides carrying side-chain modifications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service