W390607
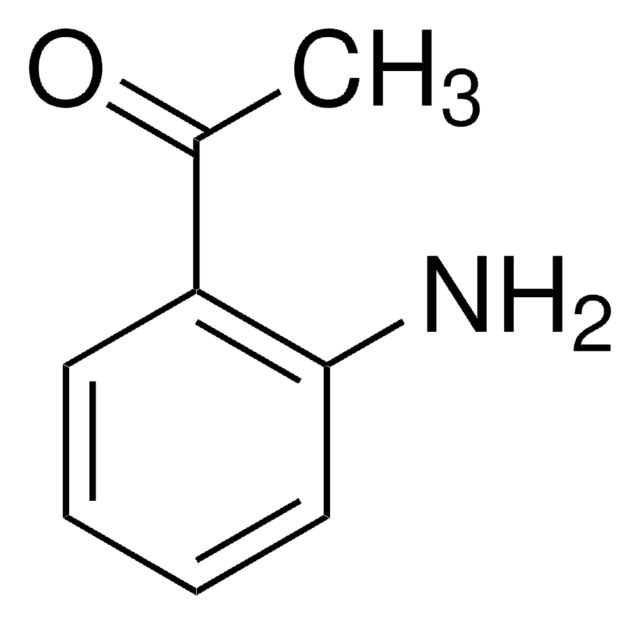
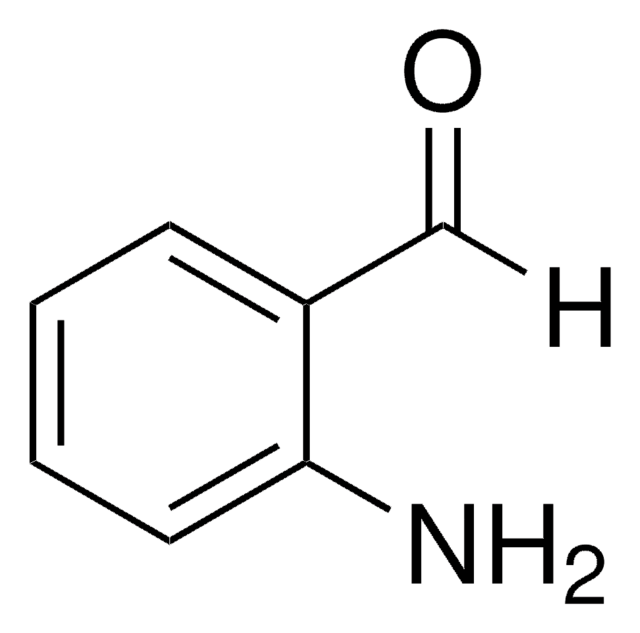
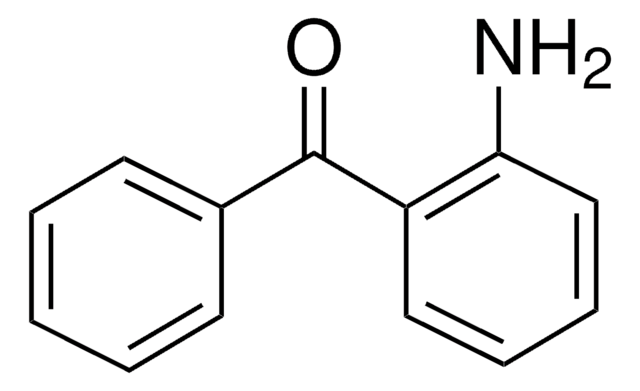
2′-Aminoacetophenone
≥98%
Synonym(s):
2-Acetylaniline
About This Item
Kosher
Recommended Products
biological source
synthetic
grade
Halal
Kosher
Assay
≥98%
refractive index
n20/D 1.614 (lit.)
bp
85-90 °C/0.5 mmHg (lit.)
density
1.112 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
grape
storage temp.
2-8°C
SMILES string
CC(=O)c1ccccc1N
InChI
1S/C8H9NO/c1-6(10)7-4-2-3-5-8(7)9/h2-5H,9H2,1H3
InChI key
GTDQGKWDWVUKTI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Disclaimer
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W390607-100G-K | 4061835513666 |
| W390607-1KG-K | 4061837807688 |
| W390607-SAMPLE-K | 4061837807695 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service