T83801
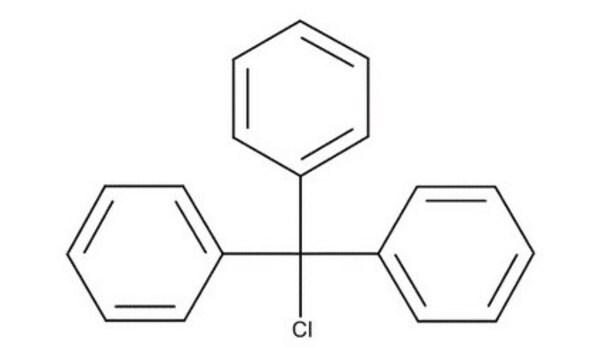
Trityl chloride
97%
Synonym(s):
Chlorotriphenylmethane, Triphenylchloromethane, Triphenylmethyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(C6H5)3CCl
CAS Number:
Molecular Weight:
278.78
Beilstein:
397363
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
230-235 °C/20 mmHg (lit.)
mp
109-112 °C (lit.)
SMILES string
ClC(c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C19H15Cl/c20-19(16-10-4-1-5-11-16,17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H
InChI key
JBWKIWSBJXDJDT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Protecting reagent for amines, alcohols, and thiols.
Trityl chloride can be used as a catalyst:
- In Ritter type synthesis of 1-amidoalkyl-2-naphtols through a one-pot three-component reaction of naphthol, aryl aldehydes and acetonitrile.
- In the synthesis of 1,2,4,5-tetrasubstituted imidazoles via a one-pot multi-component reaction of benzil with aldehydes, primary amines, and ammonium acetate.
- In the preparation of amido alkyl phenols via one-pot, multi-component reaction of phenols, aromatic aldehydes, and amides under solvent-free conditions.
- In one-pot, multi-component synthesis of substituted piperidines using aromatic aldehydes, amines, and β-ketoesters.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
One pot synthesis of 1, 2, 4, 5-tetrasubstituted-imidazoles catalyzed by trityl chloride in neutral media
Moosavi-Zare AR, et al.
Royal Society of Chemistry Advances, 4(105), 60636-60639 (2014)
Tetrahedron Letters, 48, 5017-5017 (2007)
Trityl chloride as an efficient organic catalyst for the synthesis of 1-amidoalkyl-2-naphtols in neutral media at room temperature
Khazaei A, et al.
Applied Catalysis A: General, 386(1-2), 179-187 (2010)
Greene, T.W. Wuts, P.G.M.
Protective Groups in Organic Synthesis (1991)
Solvent-free preparation of amidoalkyl phenols catalyzed by trityl chloride under neutral media
Moosavi-Zare AZ, et al.
Canadian Journal of Chemistry, 93(11), 1245-1248 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)
