T82805
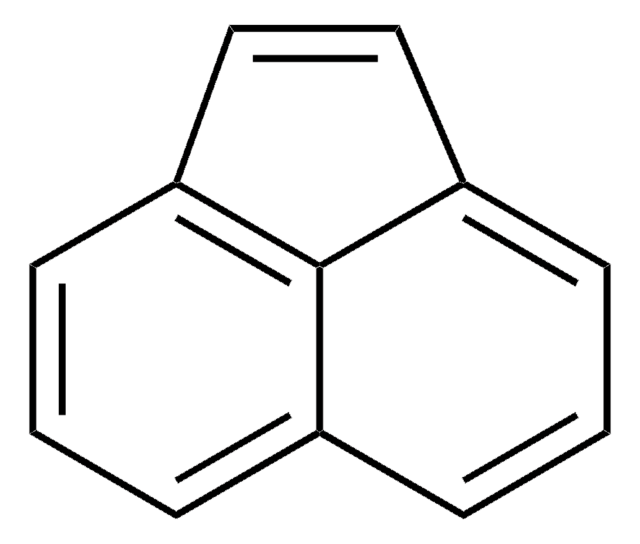
Triphenylethylene
99%
Synonym(s):
1,1,2-Triphenylethene, 1,1,2-Triphenylethylene, 1,1′,1′′-(1-Ethenyl-2-ylidene)tris[benzene], 1,2-Diphenylethenylbenzene, Benzilidenediphenylmethane, Ethene-1,1,2-triyltribenzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH=C(C6H5)2
CAS Number:
Molecular Weight:
256.34
Beilstein:
1867462
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
69-71 °C (lit.)
SMILES string
c1ccc(cc1)\C=C(/c2ccccc2)c3ccccc3
InChI
1S/C20H16/c1-4-10-17(11-5-1)16-20(18-12-6-2-7-13-18)19-14-8-3-9-15-19/h1-16H
InChI key
MKYQPGPNVYRMHI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Triphenylethylene is an aromatic hydrocarbon, which can be used as a starting material to prepare 2,2,3-triphenyloxirane by asymmetric epoxidation reaction using fluorous chiral manganese complex as a catalyst. It is also used to prepare dihydro-4,5,5-triphenyl-2(3H)-furanone by reacting with acetic anhydride in the presence of MnO2 and NaOAc.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S J Gatley et al.
International journal of radiation applications and instrumentation. Part B, Nuclear medicine and biology, 18(7), 769-775 (1991-01-01)
A triphenylethylene compound [1,1-bis(4-hydroxyphenyl)-2-iodo-2-phenylethylene; IBHPE] has been labeled by halodestannylation with 123I at a specific radioactivity of 13,200 Ci/mmol (by in vitro receptor assay) after HPLC purification. The corresponding 80mBr-labeled compound (BrBHPE), which has a 3-fold higher affinity for the
E Bignon et al.
FEBS letters, 271(1-2), 54-58 (1990-10-01)
The activation of type I (gamma), II (beta) and III (alpha) protein kinase C (PKC) subspecies by phosphatidylserine (PS) and diacylglycerol (DAG) is inhibited by micromolar concentrations of triphenylacrylonitrile (TPE) antiestrogens. TPE A (with p-hydroxy and p-diethylaminoethoxy groups on the
Synthesis of 1,1,2-triphenylethylenes and their antiproliferative effect on human cancer cell lines.
Lifang Zheng et al.
Anti-cancer drugs, 18(9), 1039-1044 (2007-08-21)
Tamoxifen analogs (1-3) and 1,1,2-triphenylethylenes (4-7) have been synthesized by the McMurry coupling reaction. Their antiproliferative effects on MCF-7 human breast-cancer cells, HO-8910 human ovarian-carcinoma cells, and (HL)-60 human promyelocytic-leukemia cells were studied by use of the colorimetric MTT assay
M Metzler et al.
American journal of clinical oncology, 14 Suppl 2, S30-S35 (1991-01-01)
The mechanisms of estrogen-induced cancer are still a matter of debate. Previous studies with stilbene estrogens and steroidal estrogens have shown that the in vitro transformation of primary Syrian hamster embryo (SHE) fibroblasts is a good experimental system for discriminating
C D van den Koedijk et al.
Biochemical pharmacology, 46(10), 1870-1872 (1993-11-17)
The binding affinity of derivatives of the triphenylethylene (TPE) antioestrogen tamoxifen and of steroidal compounds for human liver antioestrogen binding sites (AEBS) was compared with their binding affinity for rat liver AEBS. Despite the observation of some quantitative differences overall
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service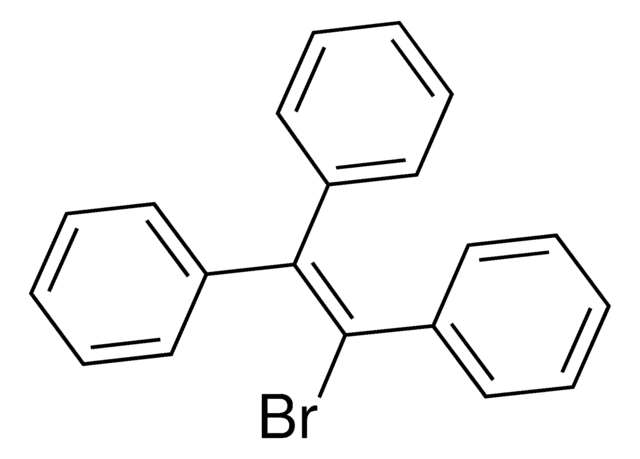

copper(I)](/deepweb/assets/sigmaaldrich/product/structures/246/823/ae3aecc5-0d68-49e8-a298-69c0d4edac35/640/ae3aecc5-0d68-49e8-a298-69c0d4edac35.png)