T48402
2,2,2-Tribromoethanol
97%
Synonym(s):
Tribromoethyl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Linear Formula:
Br3CCH2OH
CAS Number:
Molecular Weight:
282.76
Beilstein:
1733249
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
bp
92-93 °C/10 mmHg (lit.)
mp
73-79 °C (lit.)
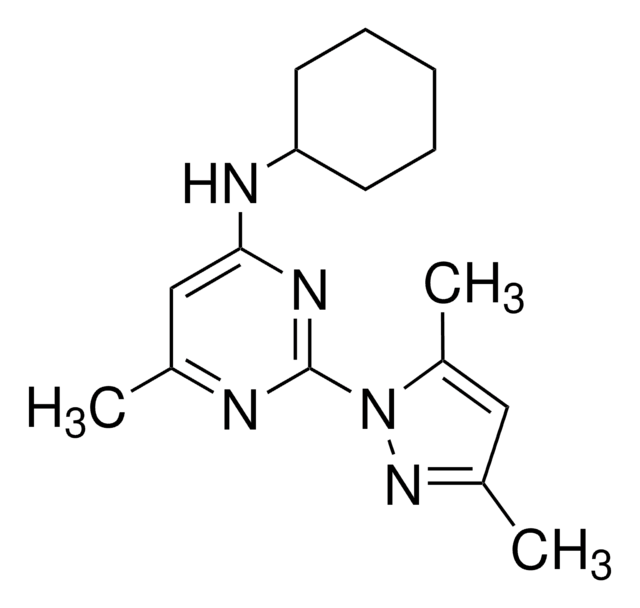
SMILES string
OCC(Br)(Br)Br
InChI
1S/C2H3Br3O/c3-2(4,5)1-6/h6H,1H2
InChI key
YFDSDPIBEUFTMI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,2,2-Tribromoethanol is generally used as an anesthetic drug for rodents.
Application
2,2,2-Tribromoethanol can be used as a functional initiator for the introduction of α-hydroxyl groups to poly(methyl methacrylate) and poly(n-butyl acrylate).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xiangjie Sun et al.
Journal of virology, 90(23), 10936-10944 (2016-09-30)
Avian influenza A H7 viruses have caused multiple outbreaks in domestic poultry throughout North America, resulting in occasional infections of humans in close contact with affected birds. In early 2016, the presence of H7N8 highly pathogenic avian influenza (HPAI) viruses
Efficacy of Tribromoethanol.
Papaioannou VE & Fox JG
Laboratory Animal Science, 43(2) (1993)
Ryo Yokoyama et al.
Stem cells translational medicine, 8(10), 1055-1067 (2019-06-04)
Clinical trials with autologous adipose-derived stem cell (AdSC) therapy for ischemic heart diseases (IHDs) are ongoing. However, little is known about combinational therapeutic effect of AdSCs and statin poly(lactic-co-glycolic) acid (PLGA) nanoparticles on the ischemic myocardium. We investigated the hypothesis
Emilio P Mottillo et al.
Molecular metabolism, 6(6), 471-481 (2017-06-06)
Fibroblast growth factor 21 (FGF21) shows great potential for the treatment of obesity and type 2 diabetes, as its long-acting analogue reduces body weight and improves lipid profiles of participants in clinical studies; however, the intracellular mechanisms mediating these effects
R J Scott Lacombe et al.
Journal of lipid research, 58(10), 2071-2081 (2017-07-12)
DHA (22:6n-3) may be derived from two dietary sources, preformed dietary DHA or through synthesis from α-linolenic acid (ALA; 18:3n-3). However, conventional methods cannot distinguish between DHA derived from either source without the use of costly labeled tracers. In the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service