All Photos(2)
About This Item
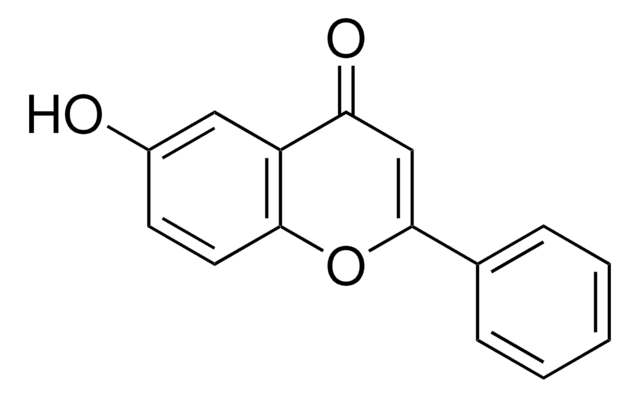
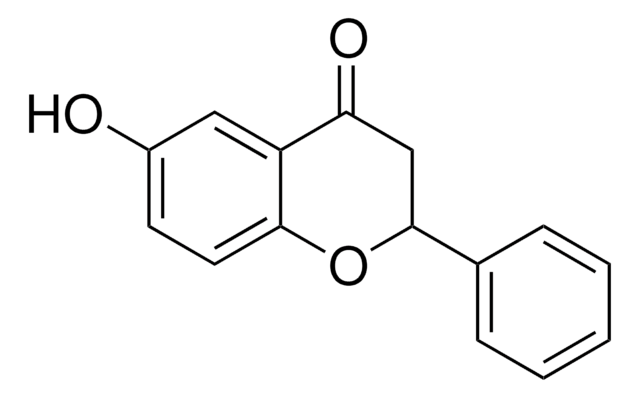
Empirical Formula (Hill Notation):
C15H10O3
CAS Number:
Molecular Weight:
238.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
mp
171-172 °C (lit.)
SMILES string
OC1=C(Oc2ccccc2C1=O)c3ccccc3
InChI
1S/C15H10O3/c16-13-11-8-4-5-9-12(11)18-15(14(13)17)10-6-2-1-3-7-10/h1-9,17H
InChI key
HVQAJTFOCKOKIN-UHFFFAOYSA-N
Gene Information
mouse ... Hexa(15211)
Looking for similar products? Visit Product Comparison Guide
Application
Reactant involved in:
Reactant involved in the synthesis of biologically active molecules including:
Involved in studies of its electrochemical properties using voltammetric methodologies
- Studies of photochemically-induced dioxygenase-type CO-release reactivity
- Phase-transfer protection and deprotection of hydroxychromones
- O-methylation with di-Me carbonate
Reactant involved in the synthesis of biologically active molecules including:
- 2-Chloropyridine derivatives for studies of antitumor agents and telomerase inhibitors
- Dihydrochromenopyrazines and chromenoquinoxalines
Involved in studies of its electrochemical properties using voltammetric methodologies
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Vasyl G Pivovarenko et al.
The journal of physical chemistry. A, 116(12), 3103-3109 (2012-03-08)
By using four labels of the 3-hydroxyflavone family displaying selective sensitivity to hydrogen bond (HB) donors and poor response to other polar molecules, we developed an approach for measuring local water concentration [H(2)O](L) (or partial volume of water: W(A) =
Arun Kumar et al.
Critical reviews in food science and nutrition, 58(11), 1791-1807 (2017-03-09)
Today, the dramatic changes in types of food consumed have led to an increased burden of chronic diseases. Therefore, the emphasis of food research is not only to ensure quality food that can supply adequate nutrients to prevent nutrition related
Cyril A Kenfack et al.
Physical chemistry chemical physics : PCCP, 14(25), 8910-8918 (2012-05-30)
The electronic transitions occurring in 4-(N,N-dimethylamino)-3-hydroxyflavone (DMAF) and 2-furanyl-3-hydroxychromone (FHC) were investigated using the TDDFT method in aprotic and protic solvents. The solvent effect was incorporated into the calculations via the PCM formalism. The H-bonding between solute and protic solvent
Sandra Irmisch et al.
Plant physiology, 180(3), 1277-1290 (2019-04-21)
The plant metabolite montbretin A (MbA) and its precursor mini-MbA are potential new drugs for treating type 2 diabetes. These complex acylated flavonol glycosides only occur in small amounts in the corms of the ornamental plant montbretia (Crocosmia × crocosmiiflora).
Biomimetic photocycloaddition of 3-hydroxyflavones: synthesis and evaluation of rocaglate derivatives as inhibitors of eukaryotic translation.
Stéphane P Roche et al.
Angewandte Chemie (International ed. in English), 49(37), 6533-6538 (2010-08-06)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service