E15701
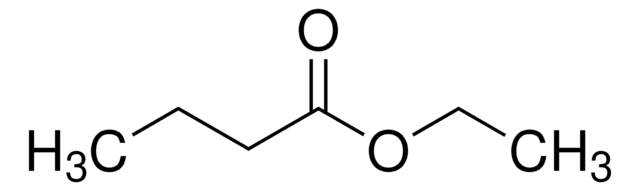
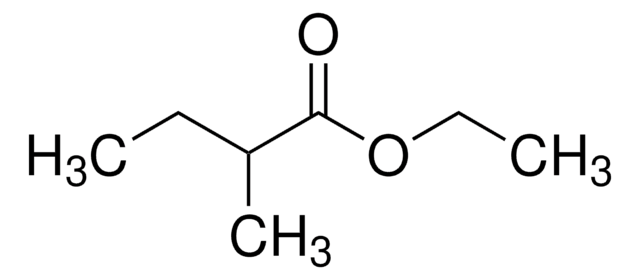
Ethyl butyrate
99%
Synonym(s):
Ethyl butanoate, Butyric acid ethyl ester
About This Item
Recommended Products
vapor density
4 (vs air)
vapor pressure
15.5 mmHg ( 25 °C)
Assay
99%
form
liquid
autoignition temp.
865 °F
refractive index
n20/D 1.392 (lit.)
bp
120 °C (lit.)
mp
−93 °C (lit.)
density
0.875 g/mL at 25 °C (lit.)
SMILES string
CCCC(=O)OCC
InChI
1S/C6H12O2/c1-3-5-6(7)8-4-2/h3-5H2,1-2H3
InChI key
OBNCKNCVKJNDBV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
78.8 °F - closed cup
Flash Point(C)
26 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Objective: To standardize a procedure for the enzymatic determination of Esterase activity using Ethyl Butyrate as a substrate.
Separation of Acetone; Acetic acid; Propionic acid; Ethyl butyrate; Ethanol; Isoamyl acetate; Isobutyric acid; 3-Methyl-2-butanol; Methyl acetate; 1-Propanol; Acetal, ≥98%, FG; 2-Methyl-1-pentanol; Butyl acetate; Ethyl propionate; 3-Pentanol; 2-Pentanol, 98%; Ethyl isobutyrate; Isobutyl acetate; Acetaldehyde; Furfural; Butyric acid; Methanol; Ethyl acetate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service