D209317
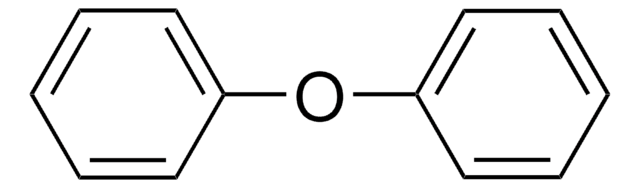
Diphenylmethane
99%
Synonym(s):
Benzylbenzene, Methylenedibenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C6H5)2CH2
CAS Number:
Molecular Weight:
168.23
Beilstein:
1904982
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
5.79 (vs air)
Quality Level
vapor pressure
<1 mmHg ( 77 °C)
Assay
99%
form
liquid
autoignition temp.
905 °F
refractive index
n20/D 1.577 (lit.)
bp
264 °C (lit.)
mp
22-24 °C (lit.)
density
1.006 g/mL at 25 °C (lit.)
SMILES string
C(c1ccccc1)c2ccccc2
InChI
1S/C13H12/c1-3-7-12(8-4-1)11-13-9-5-2-6-10-13/h1-10H,11H2
InChI key
CZZYITDELCSZES-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Diphenylmethane is an aromatic compound used as a key building block in the synthesis of elastase inhibitors.
Application
- Diphenylmethane is widely used in the synthesis of luminogens for aggregation-induced emission (AIE).
- It is used in the preparation of a polymerization initiator, diphenylmethyl potassium (DPMK).
- It is one of the precursors in the synthesis of a dendrimeric polycyclic aromatic hydrocarbon (PAH), hexakis[4-(1,1,2-triphenyl-ethenyl)phenyl]benzene.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
266.0 °F
Flash Point(C)
130 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and surface properties of amphiphilic star-shaped and dendrimer-like copolymers based on polystyrene core and poly (ethylene oxide) corona.
Francis R, et al.
Macromolecules, 36(22), 8253-8259 (2003)
Hopping of a single hole in hexakis [4-(1, 1, 2-triphenyl-ethenyl) phenyl] benzene cation radical through the hexaphenylbenzene propeller.
Rathore R, et al.
Organic Letters, 6(11), 1689-1692 (2004)
Real-time monitoring of cell apoptosis and drug screening using fluorescent light-up probe with aggregation-induced emission characteristics.
Shi H, et al.
Journal of the American Chemical Society, 134(43), 17972-17981 (2012)
Shinnosuke Hosoda et al.
Bioorganic & medicinal chemistry letters, 19(12), 3157-3161 (2009-05-19)
Based on antiviral screening of our diphenylmethane derivatives prepared as steroid substitutes, we identified a 1,1-diphenylcyclobutane analog (9) and two diethyldiphenylsilane analogs (12 and 13) as superior lead compounds with potent anti-bovine viral diarrhea virus (BVDV) activity, having 50% effective
Toshiki Nokami et al.
Journal of the American Chemical Society, 130(33), 10864-10865 (2008-07-30)
An iterative method for molecular assembly has been developed based on the cation-pool method using (trimethylsilyl)diphenylmethane as a building block. The silyl group works as both an activating group of the benzene ring in the Friedel-Crafts type reaction and an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service