D174602
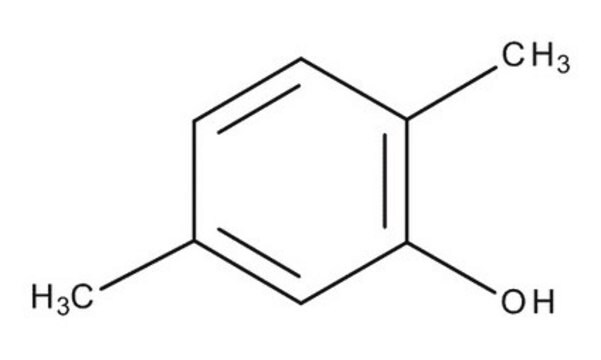
2,5-Dimethylphenol
≥99%
Synonym(s):
2-Hydroxy-p-xylene, p-Xylenol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2C6H3OH
CAS Number:
Molecular Weight:
122.16
Beilstein:
1099260
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
crystals
bp
212 °C (lit.)
mp
75-77 °C
SMILES string
Cc1ccc(C)c(O)c1
InChI
1S/C8H10O/c1-6-3-4-7(2)8(9)5-6/h3-5,9H,1-2H3
InChI key
NKTOLZVEWDHZMU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Fungicide Metabolism in Wheat: 2,5-Dimethylphenol, a breakdown product of the strobilurin fungicide mandestrobin, was studied for its metabolic pathways in wheat. This research provides insights into the environmental behavior and safety of pesticide residues in agricultural products (Ando et al., 2018).
- Tropospheric Chemistry of Dimethylphenols: The study on the mass accommodation coefficients and Henry′s law constants of 2,5-dimethylphenol underlines its significant atmospheric reactions, contributing to a better understanding of air quality and environmental chemistry (Diévart et al., 2006).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G Bieniek
Occupational and environmental medicine, 51(5), 354-356 (1994-05-01)
Phenol (87.3 mg/l), p-cresol (58.6 mg/l), o-cresol (76.9 mg/l), and 2,5-xylenol (36.7 mg/l) were detected in the urine of workers employed in the distillation of the high temperature phenolic fraction of tar (carbolic oil). The concentrations of these compounds in
Separation and determination of phenol, alpha-naphthol m- and p-, o-cresols and 2,5-xylenol, and catechol in the urine after mixed exposure to phenol, naphthalene, cresols, and xylenols.
G Bieniek et al.
British journal of industrial medicine, 43(8), 570-571 (1986-08-01)
C C Yeo et al.
Microbiology (Reading, England), 143 ( Pt 8), 2833-2840 (1997-08-01)
Pseudomonas alcaligenes NCIB 9867 (strain P25X), which grows on 2,5-xylenol and harbours the plasmid RP4, was mated with a plasmid-free derivative of Pseudomonas putida NCIB 9869, strain RA713, which cannot grow on 2,5-xylenol. Some RA713 transconjugants, initially selected on 2,5-xylenol
Chew Chieng Yeo et al.
Gene, 312, 239-248 (2003-08-12)
Pseudomonas alcaligenes NCIMB 9867 (strain P25X) produces isofunctional enzymes of the gentisate pathway that enables the degradation of xylenols and cresols via gentisate. Previous reports had indicated that one set of enzymes is constitutively expressed whereas the other set is
B J Day et al.
Research communications in chemical pathology and pharmacology, 76(1), 117-120 (1992-04-01)
Pulmonary metabolites of p-xylene, p-methylbenzyl alcohol (PMBA) and 2,5-dimethylphenol (DMP), were employed to investigate the divergent effects of p-xylene on pulmonary and hepatic metabolism. Rats were given PMBA, DMP, or 10% cremophore (control) ip daily for 3 days, and effects
Global Trade Item Number
| SKU | GTIN |
|---|---|
| D174602-100G | 4061833560600 |
| D174602-5G | 4061833560617 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service