C80857
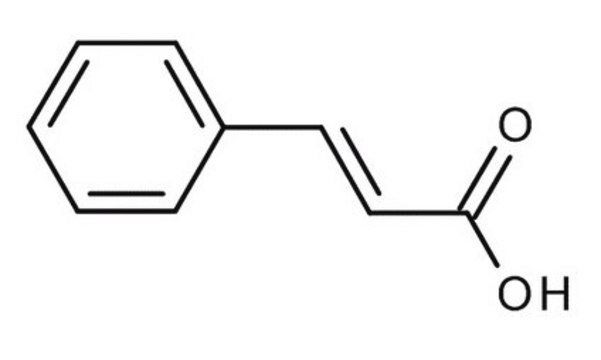
trans-Cinnamic acid
≥99%
Synonym(s):
trans-3-Phenylacrylic acid, Cinnamic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
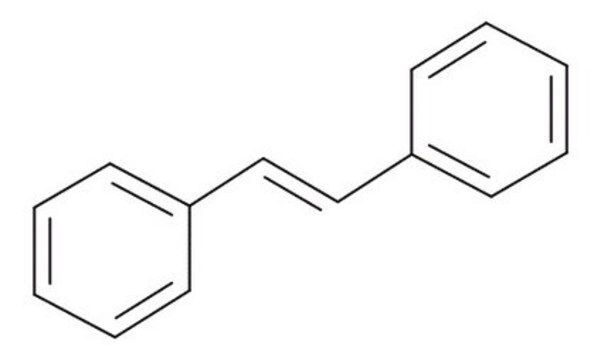
C6H5CH=CHCOOH
CAS Number:
Molecular Weight:
148.16
Beilstein:
1905952
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
form
crystals
bp
300 °C (lit.)
mp
132-135 °C (lit.)
SMILES string
OC(=O)\C=C\c1ccccc1
InChI
1S/C9H8O2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7H,(H,10,11)/b7-6+
InChI key
WBYWAXJHAXSJNI-VOTSOKGWSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
trans-Cinnamic acid can be used in the synthesis of:
- A trans-cinnamic acid hydrazide derivative with potent anti-mycobacterial activity.
- Cinnamate glycerides via homogeneous esterification reaction.
- Styrene via biocatalytic decarboxylation by plant cell cultures.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
320.0 °F - closed cup
Flash Point(C)
160 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and antimycobacterial evaluation of new trans-cinnamic acid hydrazide derivatives.
Carvalho SA, et al.
Bioorganic & Medicinal Chemistry, 18(2), 538-541 (2008)
Homogeneous catalytic esterification of glycerol with cinnamic and methoxycinnamic acids to cinnamate glycerides in solventless medium: Kinetic modeling.
Molinero L, et al.
Chemical Engineering Journal, 247(2), 174-182 (2014)
M Takemoto et al.
Chemical & pharmaceutical bulletin, 49(5), 639-641 (2001-06-01)
A novel method for producing styrenes from trans-cinnamic acids was developed. When trans-cinnamic acid was incubated with plant cell cultures at room temperature, styrene was obtained. 4-Hydroxy-3-methoxystyrene (2a), 3-nitrostyrene (2f) and furan (2g) were synthesized quantitatively.
Feng Yang et al.
Molecular pharmaceutics, 9(11), 3259-3265 (2012-09-27)
Owing to advantageous biochemical and pharmacological properties of human serum albumin (HSA), HSA-based drug carrier is playing an increasing role in the clinical setting. Since the IIA subdomain of HSA is a big hydrophobic cavity, we proposed that HSA delivers
Andrew M Lauer et al.
Organic letters, 14(19), 5138-5141 (2012-09-25)
A highly regioselective, Pd-catalyzed allylic fluorination of phosphorothioate esters is reported. This chemistry addresses several limitations of previously reported methods in which elimination and lack of reactivity were problematic. Preliminary mechanistic investigations reveal that these reactions are stereospecific and provide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service