B83606
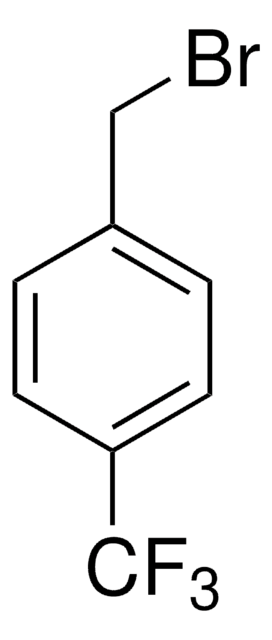
4-Methylbenzyl bromide
97%
Synonym(s):
p-Xylyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H4CH2Br
CAS Number:
Molecular Weight:
185.06
Beilstein:
507389
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
impurities
<1.5% 1,4-bis(bromomethyl)benzene
bp
218-220 °C (lit.)
mp
34-36 °C (lit.)
storage temp.
2-8°C
SMILES string
Cc1ccc(CBr)cc1
InChI
1S/C8H9Br/c1-7-2-4-8(6-9)5-3-7/h2-5H,6H2,1H3
InChI key
WZRKSPFYXUXINF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
208.4 °F - closed cup
Flash Point(C)
98 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zhouyang Li et al.
Talanta, 174, 198-205 (2017-07-26)
This paper reports novel imidazole fluorescent poly(ionic liquid) nanoparticles (FPILNs) of poly(1-[(4-methyphenyl)methyl]-3-vinyl-imidazolium bromide (poly([MVI]Br) for selective and sensitive determination of pyrogallol. An imidazole ionic liquid of 1-[(4-methyphenyl)methyl]-3-vinyl-imidazolium bromide ([MVI]Br) was synthesized and used as the only monomer to obtain poly([MVI]Br)
Ngoc-Duc Doan et al.
Journal of peptide science : an official publication of the European Peptide Society, 21(5), 387-391 (2014-11-18)
The solid-phase synthesis of azapeptides possessing a C-terminal aza-residue has been accomplished by a protocol featuring regioselective alkylation of benzhydrylidene-aza-glycinamide and illustrated by the syntheses of [aza-Lys(6)] growth-hormone-releasing peptide-6 analogs.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service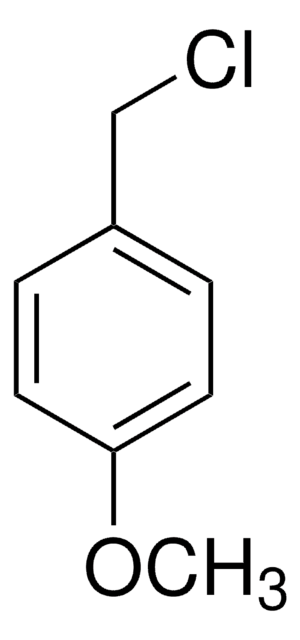
![7-Fluorobenzo[d]thiazole-2-thiol AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/803/415/c7b4e936-acdd-4efd-b0f0-2d549c0b4c7e/640/c7b4e936-acdd-4efd-b0f0-2d549c0b4c7e.png)