All Photos(1)
About This Item
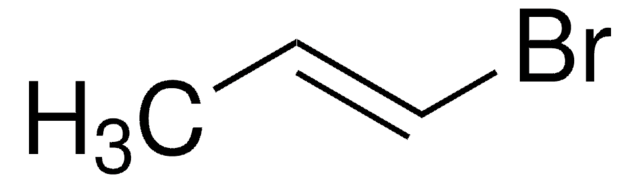
Linear Formula:
C6H5CH2CH=CH2
CAS Number:
Molecular Weight:
118.18
Beilstein:
1098501
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.511 (lit.)
bp
156-157 °C (lit.)
density
0.892 g/mL at 25 °C (lit.)
SMILES string
C=CCc1ccccc1
InChI
1S/C9H10/c1-2-6-9-7-4-3-5-8-9/h2-5,7-8H,1,6H2
InChI key
HJWLCRVIBGQPNF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Allylbenzene, also known as 3-phenylpropene, is an organic compound used as a source of the allyl group in allylation reactions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
104.0 °F - closed cup
Flash Point(C)
40 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hamid Sadeghian et al.
Journal of enzyme inhibition and medicinal chemistry, 26(2), 238-244 (2010-10-14)
A group of 2-alkoxy-5-methoxyallylbenzene were designed, synthesised and evaluated as potential inhibitors of the soybean 15-lipoxygenase (SLO) on the basis of the eugenol and esteragol structures. Compound 4d showed the best half maximal inhibitory concentration (IC₅₀) for SLO inhibition (IC₅₀ = 5.9 ± 0.6
Eric A Standley et al.
Journal of the American Chemical Society, 135(4), 1585-1592 (2013-01-16)
The synthesis and characterization of the air-stable nickel(II) complex trans-(PCy(2)Ph)(2)Ni(o-tolyl)Cl is described in conjunction with an investigation of its use for the Mizoroki-Heck-type, room temperature, internally selective coupling of substituted benzyl chlorides with terminal alkenes. This reaction, which employs a
Mitsuo Miyazawa et al.
Natural product research, 19(1), 29-36 (2005-02-11)
A methanol extract from Asiasarum heterotropoides showed a suppressive effect of the SOS-including activity on the mutagen 2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ) in the Salmonella typhimurium TA1535/pSK1002 umu test. The methanol extract was re-extracted with chloroform, butanol, and water. The chloroform fraction showed
J R Idle
Prague medical report, 106(1), 27-38 (2005-07-13)
The typical spices used in winter include nutmeg, cinnamon, clove and anise. These spices contain two groups of chemicals, the allylbenzenes and their isomers, the propenylbenzenes. It was suggested 40 years ago by Alexander Shulgin that these substances act as
Thi X Thi Luu et al.
Molecules (Basel, Switzerland), 14(9), 3411-3424 (2009-09-29)
Essential oil allylbenzenes from have been converted quickly and efficiently into the corresponding benzaldehydes in good yields by a two-step "green" reaction pathway based on a solventless alkene group isomerization by KF/Al(2)O(3) to form the corresponding 1-arylpropene and a subsequent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service