89171
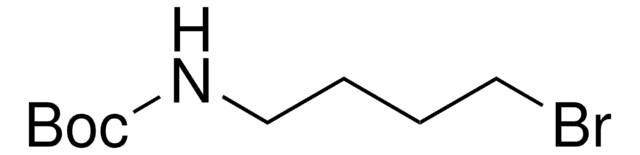
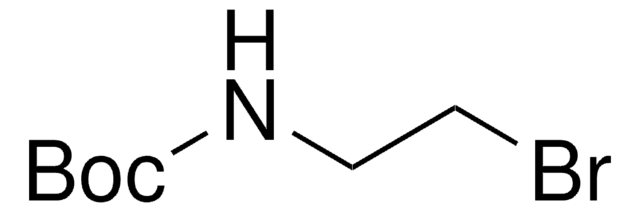
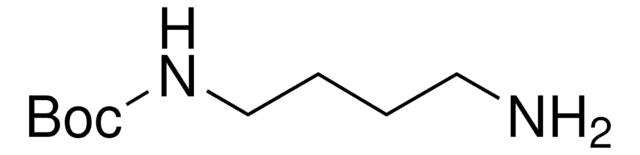
6-(Boc-amino)hexyl bromide
≥97.0% (GC)
Synonym(s):
tert-Butyl N-(6-bromohexyl)carbamate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H22BrNO2
CAS Number:
Molecular Weight:
280.20
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (GC)
form
liquid
reaction suitability
reagent type: cross-linking reagent
refractive index
n20/D 1.473
density
1.192 g/mL at 20 °C (lit.)
functional group
Boc
amine
bromo
SMILES string
O=C(NCCCCCCBr)OC(C)(C)C
InChI
1S/C11H22BrNO2/c1-11(2,3)15-10(14)13-9-7-5-4-6-8-12/h4-9H2,1-3H3,(H,13,14)
InChI key
NXQXVXILNVTMNA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Other Notes
Building block for preparing analogs of biologically active compounds
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T.H. Keller, R. Haener
Helvetica Chimica Acta, 76, 884-884 (1993)
D. Damour et al.
Bioorganic & Medicinal Chemistry Letters, 6, 1667 -1667 (1996)
Zaheer Ahmad et al.
Journal of biomaterials science. Polymer edition, 27(1), 40-54 (2015-10-23)
Tumor microenvironment-responsive nano drug delivery vehicles are gaining mounting attention in the field of biomedical sciences. The hypoxic response of the tumorous cells due to very low partial pressure of oxygen (some time less than 2.5 mm of Hg) in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service