857297
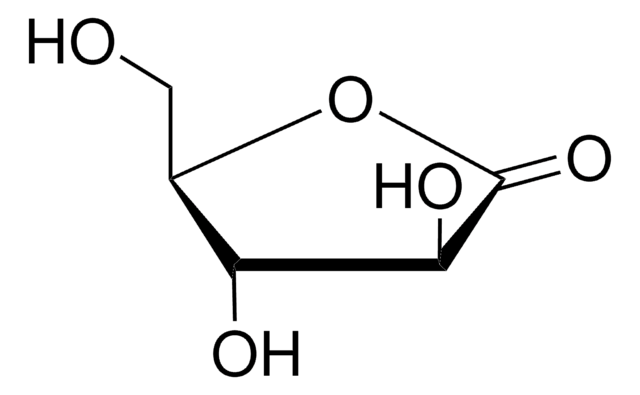
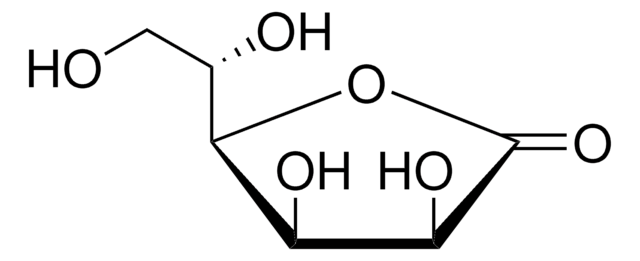
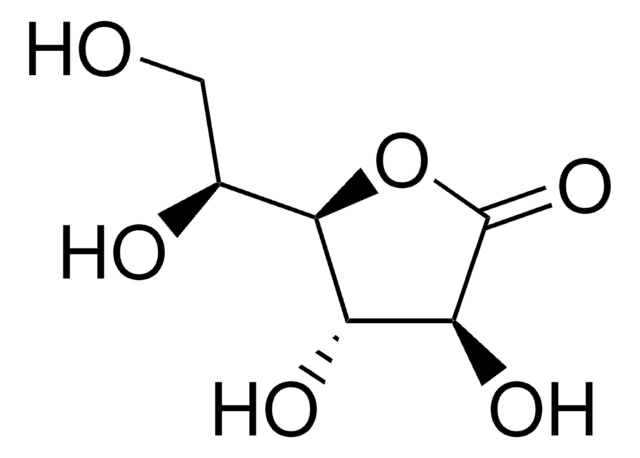
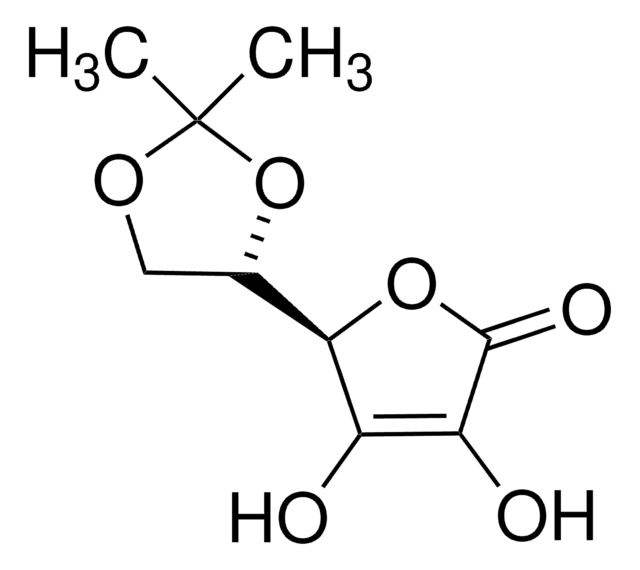
D-(+)-Ribonic γ-lactone
97%
Synonym(s):
D(+)-Ribono-1,4-lactone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8O5
CAS Number:
Molecular Weight:
148.11
Beilstein:
82057
EC Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
crystals
optical activity
[α]24/D +18°, c = 1 in H2O
mp
85-87 °C (lit.)
SMILES string
OC[C@H]1OC(=O)[C@H](O)[C@@H]1O
InChI
1S/C5H8O5/c6-1-2-3(7)4(8)5(9)10-2/h2-4,6-8H,1H2/t2-,3-,4-/m1/s1
InChI key
CUOKHACJLGPRHD-BXXZVTAOSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Important building block for chiral acyclics, cyclopentenones, and oxabicyclic systems. Also employed in studies on nonlinear optical materials.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chemistry of Materials, 5, 802-802 (1993)
Aldrichimica Acta, 22, 49-49 (1989)
Tae Woo Kim et al.
Organic letters, 6(22), 3949-3952 (2004-10-22)
[structure: see text] We describe a series of nonpolar nucleoside analogues having similar shapes and gradually increasing size. The structure of the nucleobase thymine was mimicked with toluene derivatives, replacing O2/O4 with hydrogen, fluorine, chlorine, bromine, and iodine. Glycosidic bonds
Cheng-Hung Jen et al.
Nucleosides, nucleotides & nucleic acids, 29(7), 523-534 (2010-07-01)
A thorough study for the synthesis of 1-deazauridine is described. 3-Bromo-2,6-dimethoxy-5-(beta-D-ribofuranosyl)pyridine, a synthetic precursor for 1-deazauridine, was prepared in seven steps from 2,6-dimethoxypyridine and d-ribose via the ribonolactone approach. Subsequent demethylation was unsuccessful but led to presumable anomerization and isomerization.
B A Horenstein et al.
Biochemistry, 32(28), 7089-7097 (1993-07-20)
A new approach to understanding transition-state structure is presented which involves the sequential application of experimental and computational methods. A family of experimentally determined kinetic isotope effects is fit simultaneously in a vibrational analysis to provide a geometric model of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service