761060
(Tributylphosphoranylidene)acetonitrile
97%
Synonym(s):
(Cyanomethylene)tributylphosphorane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H28NP
CAS Number:
Molecular Weight:
241.35
MDL number:
UNSPSC Code:
12352000
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.500 (lit.)
density
0.921 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
[P](=CC#N)(CCCC)(CCCC)CCCC
InChI
1S/C14H28NP/c1-4-7-11-16(14-10-15,12-8-5-2)13-9-6-3/h14H,4-9,11-13H2,1-3H3
InChI key
OZMLUMPWPFZWTP-UHFFFAOYSA-N
General description
(Tributylphosphoranylidene)acetonitrile or cyanomethylenetrimethylphosphorane (Bu3P=CHCN) is a Wittig reagent employed in the transformation of the carbonyl compounds, including aldehydes, esters, and lactones into the corresponding unsaturated nitriles.
Application
(Tributylphosphoranylidene)acetonitrile can be utilized as a reagent in the:
- Stereoselective synthesis of skytanthine and other O- and N-containing heterocycles by Mitsunobu intramolecular cycloalkylation.
- Wittig olefination of esters, lactones, N-Boc lactam, and cyclic imide to corresponding Wittig products.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Formation of heterocycles by the Mitsunobu reaction. Stereoselective synthesis of (+)-α-skytanthine
Tsunoda T, et al.
Tetrahedron Letters, 37(14), 2463-2466 (1996)
(Tributylphosphoranylidene) acetonitrile
Wyatt, Peter B
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2001)
Cyanomethylenetrimethylphosphorane, a powerful reagent for the Wittig olefination of esters, lactones and imides
Tsunoda T, et al.
Tetrahedron Letters, 41(2), 235-237 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service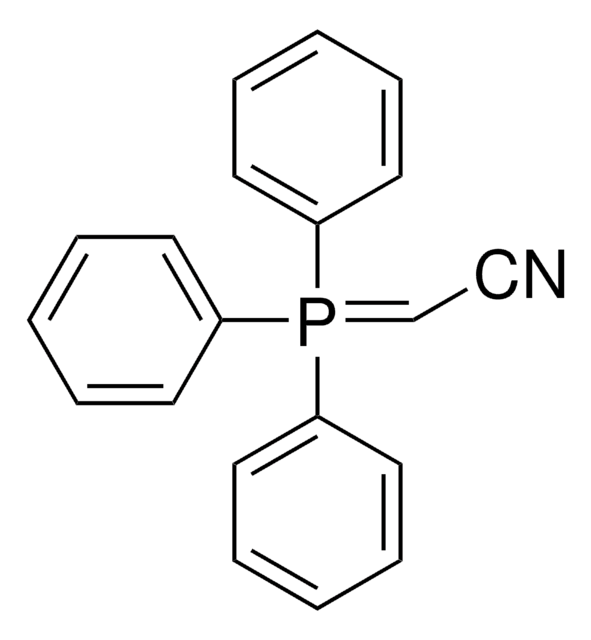


![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)