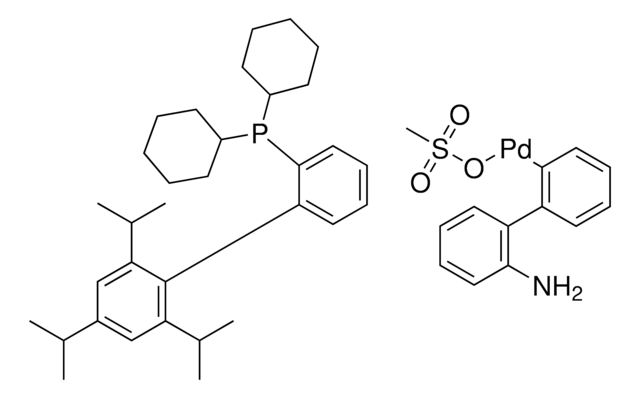
756482
P(t-Bu)3 Pd G2
Synonym(s):
Chloro[(tri-tert-butylphosphine)-2-(2-aminobiphenyl)] palladium(II)
About This Item
Recommended Products
form
solid
Quality Level
feature
generation 2
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
167-170 °C (decomposition)
functional group
phosphine
SMILES string
NC1=C(C2=CC=CC=C2[Pd]Cl)C=CC=C1.CC(C)(C)P(C(C)(C)C)C(C)(C)C
InChI
1S/C12H10N.C12H27P.ClH.Pd/c13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-10(2,3)13(11(4,5)6)12(7,8)9;;/h1-6,8-9H,13H2;1-9H3;1H;/q;;;+1/p-1
InChI key
ZVSLIOFJVMRWHJ-UHFFFAOYSA-M
General description
Application
- Synthesis of sterically hindered biaryls (tetra-ortho-substituted), via cross-coupling reactions of aryl chlorides.
- Stille cross-couplings reactions of aryl chloride.
- Synthesis of chloropeptin I, via Stille cross-coupling reaction.
- Heck reaction.
- Negishi cross-coupling reactions.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form. Once activated by base under the reaction conditions they become sensitive to air. To best enable scale-up success, the use of standard Schlenk technique is recommended.
All contents in the foil bag are weighed, plated, packed, and sealed in a glove box under nitrogen.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)