667234
Chloro(pentamethylcyclopentadienyl)(cyclooctadiene)ruthenium(II)
Synonym(s):
Cp*RuCl(cod), 1,5-Cyclooctadiene, ruthenium complex, Chloro(1,5-cyclooctadiene)(η5-pentamethylcyclopentadienyl)ruthenium, Chloro(1,5-cyclooctadiene)(pentamethylcyclopentadienyl)ruthenium
About This Item
Recommended Products
reaction suitability
core: ruthenium
reagent type: catalyst
Quality Level
mp
143-147 °C
storage temp.
−20°C
SMILES string
Cl[Ru].C1CC=CCCC=C1.C[C]2[C](C)[C](C)[C](C)[C]2C
InChI
1S/C10H15.C8H12.ClH.Ru/c1-6-7(2)9(4)10(5)8(6)3;1-2-4-6-8-7-5-3-1;;/h1-5H3;1-2,7-8H,3-6H2;1H;/q;;;+1/p-1/b;2-1-,8-7-;;
InChI key
MQMQNIQJGNBEMG-ONEVTFJLSA-M
Application
It can be used:
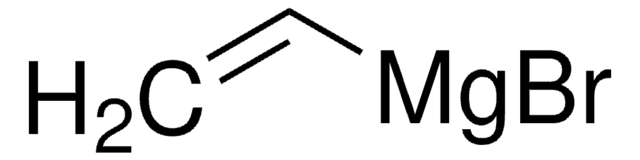
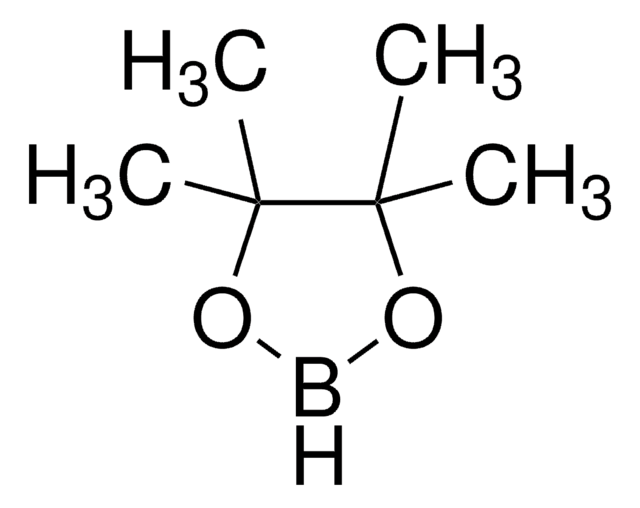
- To catalyze cyclotrimerization of alkynylboronates, propargyl alcohols, and terminal alkynes to form arylboronate, which in turn undergoes palladium(II)-catalyzed carbonylation to form highly substituted phthalides.
- To catalyze C-C coupling of norbornenes and norbornadiene with alkynes to form [2 + 2] cycloadducts.
- In combination with 2-diphenylphosphinoethylamine-potassium tertiary butoxide to form a ternary catalyst system that can catalyze fast racemization of chiral non-racemic sec-alcohols.
- To synthesize new organoruthenium complexes with phosphorus-based ligands such as bis(phosphino)amines.
- To catalyze the addition of organic disulfides to alkenes leading to vic-dithioethers.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Water-react 2
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Dichlorotriphenylphosphine[bis(2-(ethylthio)ethyl)amine]ruthenium(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/355/871/c9ac8b63-9a77-4c66-b545-cb7c41673aa4/640/c9ac8b63-9a77-4c66-b545-cb7c41673aa4.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)