666548
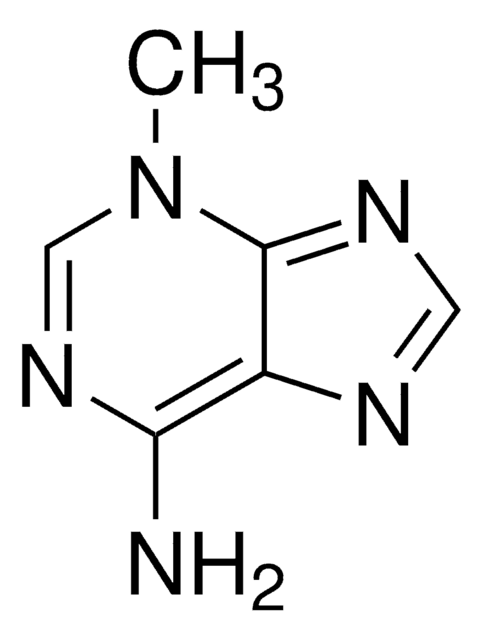
7-Methyladenine
97%
Synonym(s):
6-Amino-7-methylpurine, 7-Methyl-7H-purin-6-amine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H7N5
CAS Number:
Molecular Weight:
149.15
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
346-350 °C
SMILES string
Cn1cnc2ncnc(N)c12
InChI
1S/C6H7N5/c1-11-3-10-6-4(11)5(7)8-2-9-6/h2-3H,1H3,(H2,7,8,9)
InChI key
HCGHYQLFMPXSDU-UHFFFAOYSA-N
Application
<ul>
<li><strong>Biomarkers of Cigarette Smoking and DNA Methylating Agents:</strong> Study on 7-Methyladenine highlights its role as a biomarker of DNA damage from exposure to methylating agents (Harroun et al., 2017).</li>
</ul>
<li><strong>Biomarkers of Cigarette Smoking and DNA Methylating Agents:</strong> Study on 7-Methyladenine highlights its role as a biomarker of DNA damage from exposure to methylating agents (Harroun et al., 2017).</li>
</ul>
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B Tudek et al.
Acta biochimica Polonica, 46(3), 785-799 (2000-03-04)
The most abundant lesion formed in DNA upon modification with methylating agents 7-methylguanine, under alkaline conditions is converted into 2,6-diamino-4-hydroxy-5N-methyl-formamidopyrimidine (Fapy-7MeGua). We have previously shown that treatment of dimethylsulfate methylated DNA with NaOH creates mutagenic base derivatives leading to a
H G Mandel et al.
Analytical biochemistry, 217(2), 292-297 (1994-03-01)
We have developed a procedure for isolating and quantifying 7-methyladenine from rat urine following the administration to the rat of methylating agents, such as dimethylnitrosamine. Urinary 7-methyladenine and its trideutero isomer, added as an internal standard, were precipitated with silver
Shinji Tokuda et al.
Bioscience, biotechnology, and biochemistry, 76(4), 828-830 (2012-04-10)
Adenine had a concentration-dependent relaxation action on the phenylephrine-contracted aorta ring, with an EC(50) value of 0.40±0.12 mM. This effect was also observed in the endothelium-denuded aorta. Among the adenine analogues, N-methyladenine and benzimidazole still evoked an apparent relaxation effect
H G Mandel et al.
Carcinogenesis, 15(7), 1393-1398 (1994-07-01)
Earlier studies showed that urine of rats which had been injected with the methylating agent N-[3H-methyl]-N-nitrosourea contained a previously undetected metabolic product, 7-[3H-methyl]adenine. This methylpurine, undoubtedly derived from alkylation of nucleic acids followed by depurination, was not labeled when 14C-methyl-labeled
H G Mandel et al.
Carcinogenesis, 10(4), 757-762 (1989-04-01)
Relatively simple and rapid analytical procedures involving two sequential HPLC separations were developed for the isolation of methylated purines in the urine of rats administered radiolabeled methylating carcinogens. Following a dose of [3H]N-methyl-N-nitrosourea (MNU), 7-methyl-adenine (m7Gua) was detected by chromatography
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service