597139
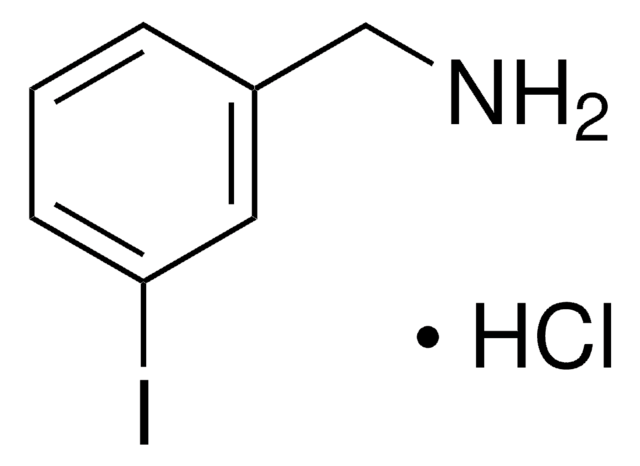
4-Iodobenzylamine hydrochloride
95%
Synonym(s):
(4-Iodophenyl)methanamine hydrochloride, p-Iodobenzylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
IC6H4CH2NH2 · HCl
CAS Number:
Molecular Weight:
269.51
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
299-303 °C (lit.)
functional group
amine
iodo
SMILES string
Cl[H].NCc1ccc(I)cc1
InChI
1S/C7H8IN.ClH/c8-7-3-1-6(5-9)2-4-7;/h1-4H,5,9H2;1H
InChI key
GBJMURRFWZREHE-UHFFFAOYSA-N
General description
4-Iodobenzylamine hydrochloride can be synthesized in three steps from 4-iodobenzoic acid.
Application
4-Iodobenzylamine hydrochloride can react with methyl 4-bromomethyl-3-methoxycarbonyl cinnamate in the presence of triethylamine to give the corresponding cyclic amide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
"Design, synthesis, and evaluation of isoindolinone-hydroxamic acid derivatives as histone deacetylase (HDAC) inhibitors"
Lee S, et al.
Bioorganic & Medicinal Chemistry, 17(27), 4895-4900 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service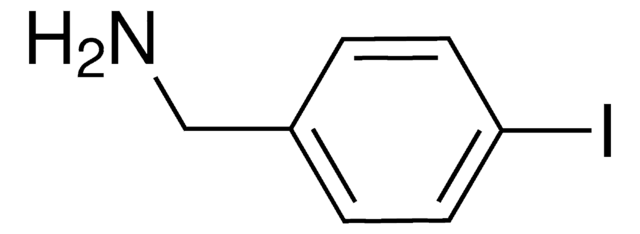


![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)