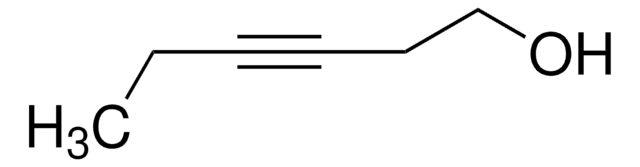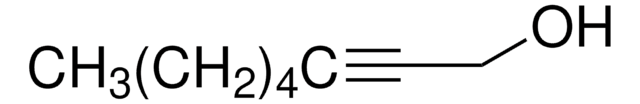All Photos(1)
About This Item
Linear Formula:
CH3(CH2)3C≡CCH2CH2OH
CAS Number:
Molecular Weight:
126.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.4569 (lit.)
density
0.880 g/mL at 25 °C (lit.)
SMILES string
CCCCC#CCCO
InChI
1S/C8H14O/c1-2-3-4-5-6-7-8-9/h9H,2-4,7-8H2,1H3
InChI key
LRZGRGVRZSDRTK-UHFFFAOYSA-N
Related Categories
General description
3-Octyn-1-ol is a homopropargylic alcohol that can be prepared from 3-butyn-1-ol and 1-bromobutane.
Application
3-Octyn-1-ol may be used in the preparation of:
- (3Z)-octen-1-ol
- 7-octyn-1-ol
- 3-cis-octenoic acid
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Selective cleavage of dimethylhydrazones to the carbonyl compounds using silica gel and its application in the synthesis of (Z)-9-tetradecenyl acetate.
Mitra RB and Reddy GB.
Synthesis, 1989(09), 694-698 (1989)
Peter Witzgall et al.
Journal of chemical ecology, 31(12), 2923-2932 (2005-12-21)
Analysis of extracts of sex pheromone glands of grapevine moth females Lobesia botrana showed three previously unidentified compounds, (E)-7-dodecenyl acetate and the (E,E)- and (Z,E)-isomers of 7,9,11-dodecatrienyl acetate. This is the first account of a triply unsaturated pheromone component in
Pd/CaCO3 in liquid poly (ethylene glycol)(PEG): an easy and efficient recycle system for partial reduction of alkynes to cis-olefins under a hydrogen atmosphere.
Chandrasekhar S, et al.
Tetrahedron Letters, 45(11), 2421-2423 (2004)
Dongyan Zhang et al.
The Journal of biological chemistry, 277(11), 9127-9132 (2002-01-10)
The degradation of unsaturated fatty acids by beta-oxidation involves Delta(3),Delta(2)-enoyl-CoA isomerases (enoyl-CoA isomerases) that catalyze 3-cis --> 2-trans and 3-trans --> 2-trans isomerizations of enoyl-CoAs and the 2,5 --> 3,5 isomerization of dienoyl-CoAs. An analysis of rat liver enoyl-CoA isomerases
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service