All Photos(2)
About This Item
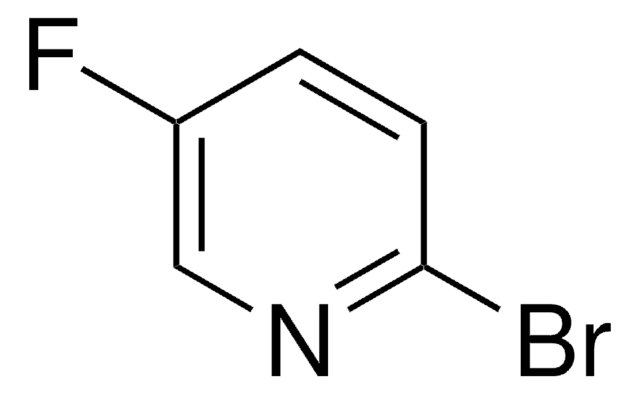
Empirical Formula (Hill Notation):
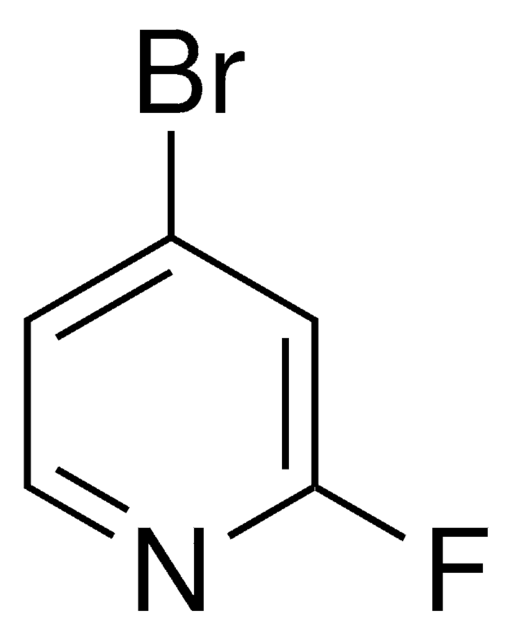
C5H3BrFN
CAS Number:
Molecular Weight:
175.99
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
refractive index
n20/D 1.5325 (lit.)
bp
162-164 °C/750 mmHg (lit.)
density
1.71 g/mL at 25 °C (lit.)
functional group
bromo
fluoro
SMILES string
Fc1ccc(Br)cn1
InChI
1S/C5H3BrFN/c6-4-1-2-5(7)8-3-4/h1-3H
InChI key
MYUQKYGWKHTRPG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
163.4 °F - closed cup
Flash Point(C)
73 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Andrew Sutherland et al.
The Journal of organic chemistry, 68(6), 2475-2478 (2003-03-15)
Two racemic fluoropyridine analogues 4 and 5 of the potent nicotinic agonist UB-165 have been synthesized. Halogenated pyridines 7 and 12 provided the organometallic reagents needed for the Negishi and Suzuki coupling reactions used for the preparation of 4 and
Andrew Sutherland et al.
The Journal of organic chemistry, 68(8), 3352-3355 (2003-04-12)
5-bromo-2-fluoro-3-pyridylboronic acid (3) was prepared in high yield by ortho-lithiation of 5-bromo-2-fluoropyridine (1), followed by reaction with trimethylborate. Suzuki reaction of 3 with a range of aryl iodides gave 3-monosubstituted 5-bromo-2-fluoropyridines 4 in excellent yields. A second Suzuki reaction utilizing
Aromatic fluorine compounds. XI. Replacement of chlorine by fluorine in halopyridines.
Finger GC, et al.
The Journal of Organic Chemistry, 28(6), 1666-1668 (1963)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service