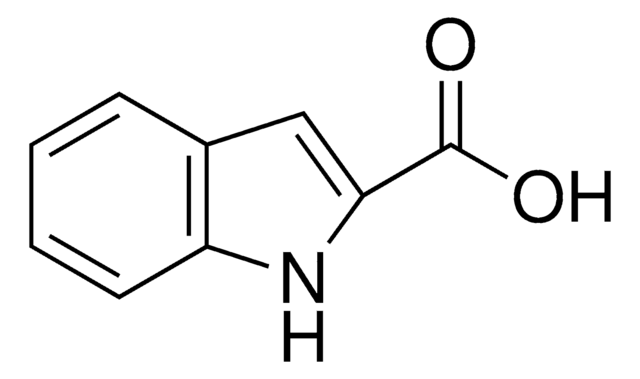
511129
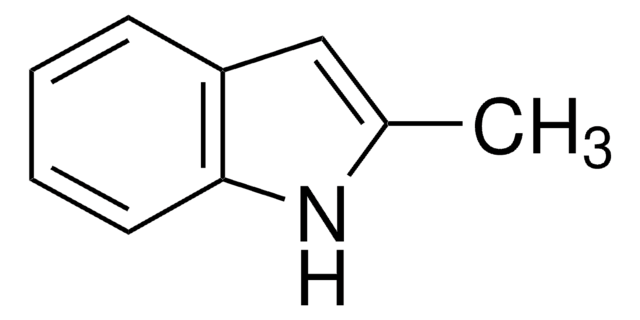
1-Methylindole-2-carboxaldehyde
97%
Synonym(s):
2-Formyl-1-methylindole, NSC 106285
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H9NO
CAS Number:
Molecular Weight:
159.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
81-85 °C (lit.)
functional group
aldehyde
SMILES string
Cn1c(C=O)cc2ccccc12
InChI
1S/C10H9NO/c1-11-9(7-12)6-8-4-2-3-5-10(8)11/h2-7H,1H3
InChI key
IBNGPIOSWCMJGG-UHFFFAOYSA-N
Application
1-Methylindole-2-carboxaldehyde may be used in the synthesis of indole-based melatonin analog hydrazone derivatives and (E)-5-((benzyloxy)methyl)-5-(((tert-butyldiphenylsilyl)oxy)methyl)-3-((1-methyl-1H-indol-2-yl)methylene)dihydrofuran-2(3H)-one.
Reactant for preparation of:
- Deazapurine isosteres from acylindoles via pyridine ring annulation
- 2,4-dichlorocinnamohydroxamic acid analogs for enhancing pharmacokinetics of botulinum neurotoxin serotype A protease inhibitors
- Tetrahydrocarbazoles via organocatalytic cascade Friedel-Crafts alkylation/Michael addition/aromatization reaction
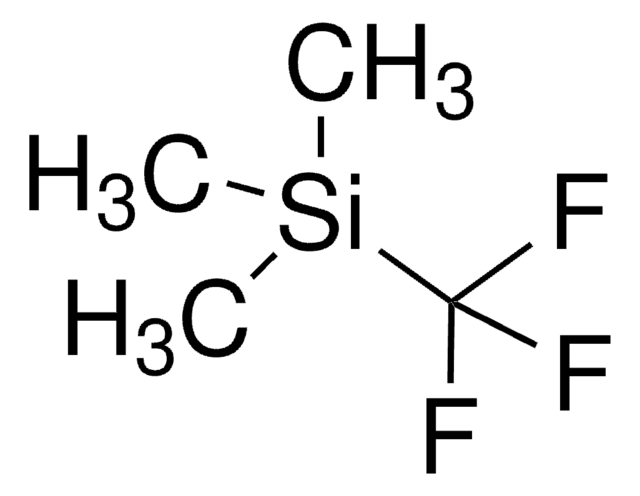
- Azides, imines and amines via flow processes of amines and trimethylsilyl azide and aza-Wittig reaction
- 3-indolylpyridinedicarbonitriles as anti-inflammatory agents
- Bis(indolyl)methanes as antimicrobial and antioxidant agents
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sibel Suzen et al.
Journal of enzyme inhibition and medicinal chemistry, 28(6), 1143-1155 (2012-09-22)
Melatonin (MLT) is a strong free-radical scavenger, which protects the body from the effects of oxidants. In recent years, MLT have been described resulting in much attention in the development of synthetic compounds possessing. As a part of our ongoing
Noga Gal et al.
Chembiochem : a European journal of chemical biology, 12(15), 2331-2340 (2012-10-30)
N-methyl-substituted diacylglycerol-indololactones (DAG-indololactones) are newly synthesized effectors of protein kinase C (PKC) isoforms and exhibit substantial selectivity between RasGRP3 and PKCα. We present a comprehensive analysis of membrane interactions and biological activities of several DAG-indololactones. Translocation and binding activity assays
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service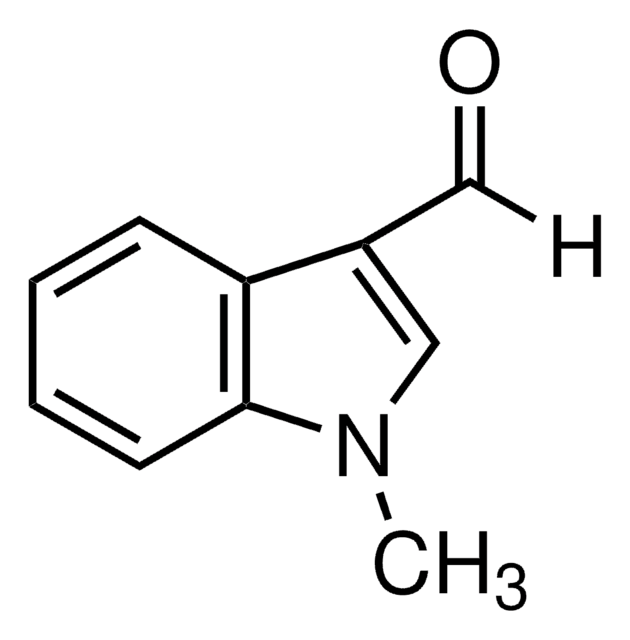

![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)