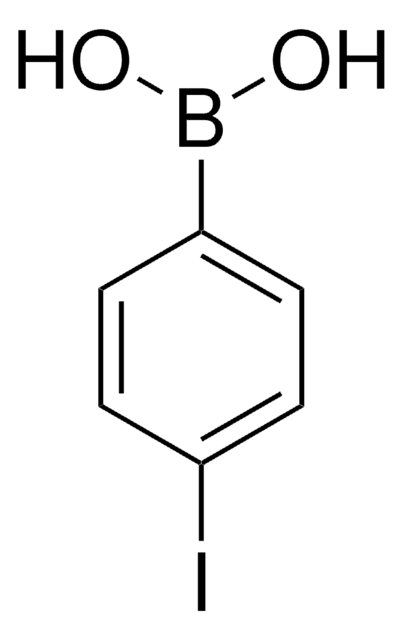
473804
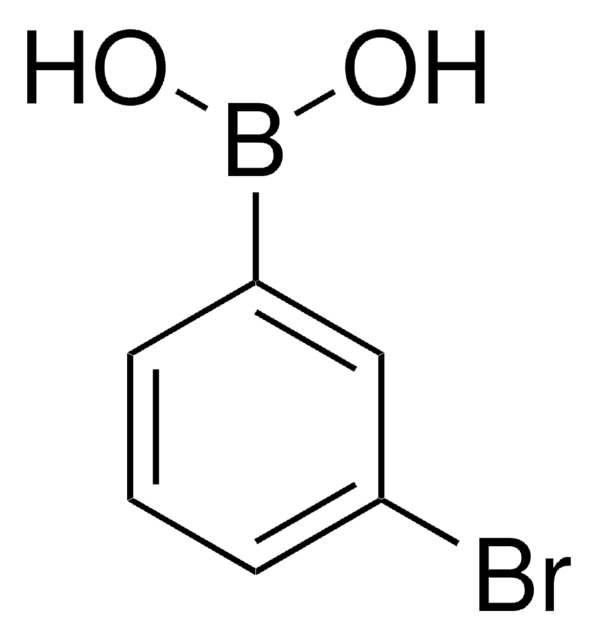
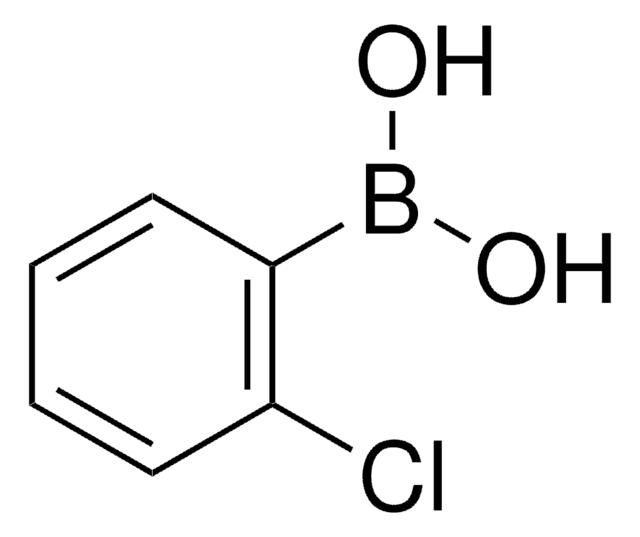
2-Bromophenylboronic acid
≥95.0%
Synonym(s):
o-Bromophenylboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
BrC6H4B(OH)2
CAS Number:
Molecular Weight:
200.83
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
113 °C (lit.)
greener alternative category
SMILES string
OB(O)c1ccccc1Br
InChI
1S/C6H6BBrO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4,9-10H
InChI key
PLVCYMZAEQRYHJ-UHFFFAOYSA-N
General description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalysis. Find details here.
Application
Catalyzes the formation of amide bonds from amines and carboxylic acids.
Used to promote greener amidations of carboxylic acids and amines in catalytic amounts. This technology avoids the requirement of preactivation of the carboxylic acid or use of coupling reagents.
Direct Amidation of Carboxylic Acids Catalyzed by ortho-Iodo Arylboronic Acids: Catalyst Optimization, Scope, and Preliminary Mechanistic Study Supporting a Peculiar Halogen Acceleration Effect
Direct Amidation of Carboxylic Acids Catalyzed by ortho-Iodo Arylboronic Acids: Catalyst Optimization, Scope, and Preliminary Mechanistic Study Supporting a Peculiar Halogen Acceleration Effect
Other Notes
Contains varying amounts of anhydride
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yae In Cho et al.
Inorganic chemistry, 58(19), 12689-12699 (2019-09-10)
We report syntheses and H2 activation involving model complexes of mono-iron hydrogenase (Hmd) derived from acyl-containing pincer ligand precursors bearing thioether (CNS
Articles
We are proud to offer a number of products used in catalytic amidation technology.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service