441643
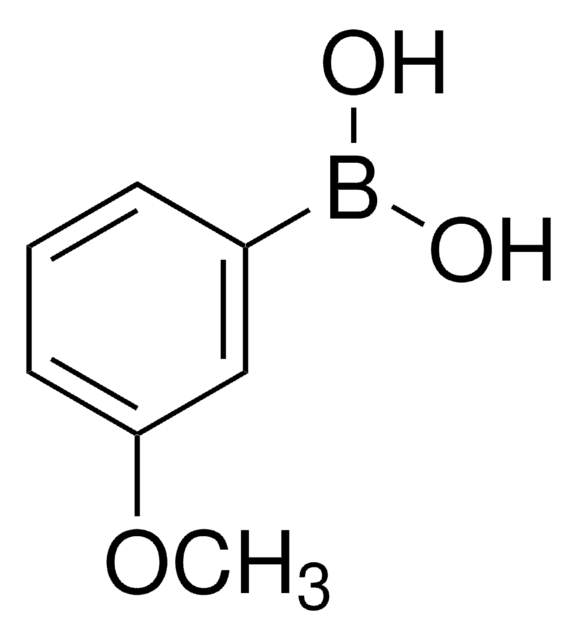
3-Fluorophenylboronic acid
≥95.0%
Synonym(s):
(3-Fluorophenyl-1-yl)boronic acid, 3-Fluorobenzeneboronic acid, m-Fluorophenylboronic acid, m-fluoro-Benzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
FC6H4B(OH)2
CAS Number:
Molecular Weight:
139.92
Beilstein:
3030632
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
mp
214-218 °C (lit.)
functional group
fluoro
SMILES string
OB(O)c1cccc(F)c1
InChI
1S/C6H6BFO2/c8-6-3-1-2-5(4-6)7(9)10/h1-4,9-10H
InChI key
KNXQDJCZSVHEIW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Recently used to make novel liquid crystalline fluorobiphenylcyclohexenes and difluoroterphenyls by palladium-catalyzed cross-couplings also used in the synthesis of o-phenylphenols as potent leukotriene B4 receptor agonists.
Other Notes
Contains varying amounts of anhydride
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Liq. Cryst., 18, 1-1 (1995)
o-phenylphenols: potent and orally active leukotriene B4 receptor antagonists.
M J Sofia et al.
Journal of medicinal chemistry, 36(24), 3978-3981 (1993-11-26)
Journal of Materials Chemistry, 5, 2239-2239 (1995)
Chihiro Takei et al.
Chemical & pharmaceutical bulletin, 66(4), 368-374 (2018-04-03)
Previous studies have shown that reversible chemical bond formation between phenylboronic acid (PBA) and 1,3-diol can be utilized as the driving force for the preparation of layer-by-layer (LbL) films. The LbL films composed of a PBA-appended polymer and poly(vinyl alcohol)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service