420964
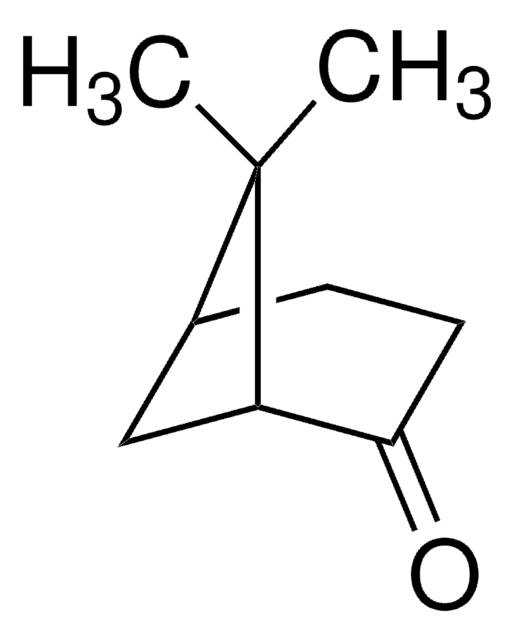
(1S)-(+)-Ketopinic acid
99%
Synonym(s):
(1S)-7,7-Dimethyl-2-oxobicyclo[2.2.1]heptane-1-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H14O3
CAS Number:
Molecular Weight:
182.22
Beilstein:
4180005
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
optical activity
[α]23/D +58°, c = 1 in chloroform
mp
237-239 °C (lit.)
SMILES string
CC1(C)C2CCC1(C(O)=O)C(=O)C2
InChI
1S/C10H14O3/c1-9(2)6-3-4-10(9,8(12)13)7(11)5-6/h6H,3-5H2,1-2H3,(H,12,13)/t6-,10+/m1/s1
InChI key
WDODWBQJVMBHCO-LDWIPMOCSA-N
Looking for similar products? Visit Product Comparison Guide
General description
(1S)-(+)-Ketopinic acid is a chiral ketone.
Application
(1S)-(+)-Ketopinic acid may be used to prepare:
- Chiral phosphine-oxazoline ligands, which can used in asymmetric palladium-catalyzed Heck reaction of aryl or alkenyl triflates with cyclic alkenes.
- A chiral imino-phosphine ligand, which can be used in palladium-catalyzed asymmetric Diels–Alder reactions to prepare six-membered carbocycles.
- A chiral iminopyridine ligand, which can be used in copper-catalyzed Henry (nitro aldol) reaction between nitromethane and o-anisol to form the corresponding β-hydroxynitroalkane.
Used to prepare a new chiral oxazolidone auxiliary and a ketopinic derivatized polymer used for the deracemization of amines. Starting material for the synthesis of homochiral 2,10-camphanediols. Employed in the formation of a chiral Schiff base precursor to diethyl (S)-α-amino-α-alkyl phosphonates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yan, T.-H. et al.
Tetrahedron Letters, 32, 4959-4959 (1991)
Deng, J. et al.
Synthesis, 963-963 (1991)
Chiral P, N-ligands based on ketopinic acid in the asymmetric Heck reaction.
Gilbertson SR and Fu Z.
Organic Letters, 3(2), 161-164 (2001)
Calmes, M. et al.
Tetrahedron Asymmetry, 5, 817-817 (1994)
Palladium-catalyzed asymmetric Diels-Alder reactions with novel chiral imino-phosphine ligands.
Hiroi K and Watanabe K.
Tetrahedron Asymmetry, 12(22), 3067-3071 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service