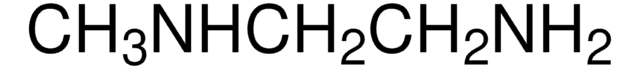39030
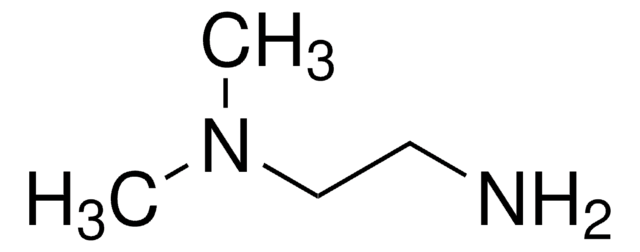
N,N-Dimethylethylenediamine
≥98.0% (GC)
Synonym(s):
2-(Dimethylamino)ethylamine, DMEN
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
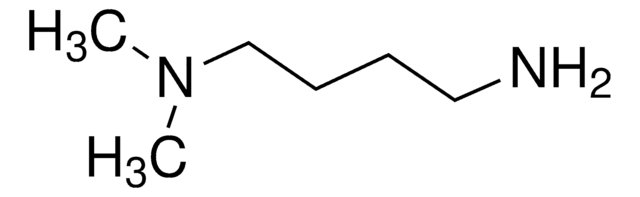
(CH3)2NCH2CH2NH2
CAS Number:
Molecular Weight:
88.15
Beilstein:
605279
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (GC)
form
liquid
refractive index
n20/D 1.426 (lit.)
n20/D 1.427
bp
104-106 °C (lit.)
density
0.807 g/mL at 20 °C (lit.)
functional group
amine
SMILES string
CN(C)CCN
InChI
1S/C4H12N2/c1-6(2)4-3-5/h3-5H2,1-2H3
InChI key
DILRJUIACXKSQE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N,N-Dimethylethylenediamine is a bidentate ligand that can be used:
- To synthesize Schiff base ligands to prepare their zinc alkyl and zinc alkoxide complexes that initiate ring-opening polymerization of lactides.
- As a ligand to prepare copper azido polymers.
- In the synthesis of naphthalimides such as amonafide, an antitumor agent.
- As the receptor in building fluorescent signaling systems to perturb the photoinduced intramolecular electron transfer (PET) process from the nitrogen lone-pair to the fluorophore.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
53.1 °F - closed cup
Flash Point(C)
11.7 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Intramolecular Aryne-Furan Cycloadditions for the Synthesis of Anticancer Naphthalimides.
Prevost S, et al.
The Journal of Organic Chemistry, 83(8), 4871-4881 (2018)
Pei Zhao et al.
Langmuir : the ACS journal of surfaces and colloids, 35(17), 5779-5786 (2019-01-24)
Nosocomial infections are a major problem in medical health care. To solve this problem, a series of antimicrobial waterborne paints were prepared by using antimicrobial hyperbranched (HB) emulsifiers. The HB-emulsifiers were prepared by polymerizing AB2 monomers obtained in a one-step
Perturbation of the PET process in fluorophore? spacer? receptor systems through structural modification: Transition metal induced fluorescence enhancement and selectivity.
Bag B and Bharadwaj P K
The Journal of Physical Chemistry B, 109(10), 4377-4390 (2005)
Synthesis, structures, and magnetic behavior of a series of copper (II) azide polymers of Cu4 building clusters and isolation of a new hemiaminal ether as the metal complex.
Mukherjee S, et al.
Inorganic Chemistry, 50(8), 3621-3631 (2011)
Ring-opening polymerization of lactides initiated by zinc alkoxides derived from NNO-tridentate ligands.
Chen H Y, et al.
Macromolecules, 39(11), 3745-3752 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service