All Photos(1)
About This Item
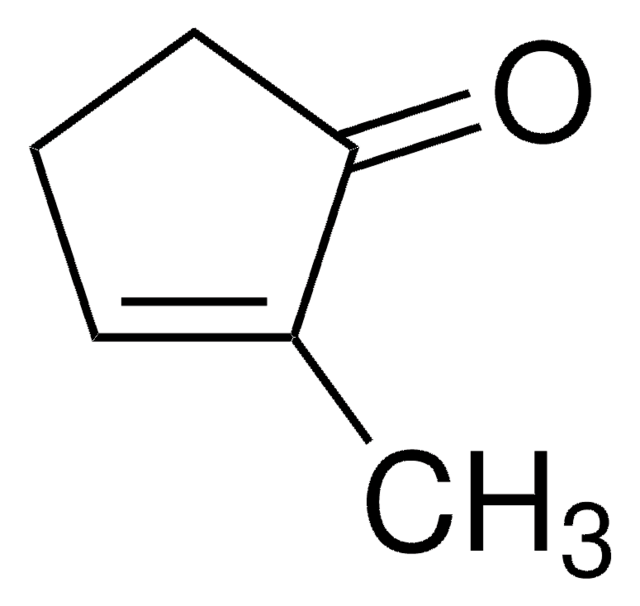
Linear Formula:
(CH3)2C5H6(=O)
CAS Number:
Molecular Weight:
112.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
refractive index
n20/D 1.433 (lit.)
bp
143-145 °C (lit.)
density
0.894 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
CC1(C)CCCC1=O
InChI
1S/C7H12O/c1-7(2)5-3-4-6(7)8/h3-5H2,1-2H3
InChI key
FTGZMZBYOHMEPS-UHFFFAOYSA-N
General description
2,2-Dimethylcyclopentanone is a ketone. Synthesis of various C-2 substituted vitamin D derivatives with a 2,2-dimethylcyclopentanone unit in side chains has been reported. Regioselective synthesis of 2,2-dimethylcyclopentanone, via its enolate precursor regioselectively obtained using the 2-pyrrolidone magnesium salt, has been reported.
Application
2,2-Dimethylcyclopentanone enolate may be used as starting reagent in the enantioselective synthesis of chiral phosphines belonging to the P-aryl-2-phosphabicyclo[3.3.0]octane family (PBO). It may be used in the synthesis of:
- 2,6,6-trimethyl-2-azaspiro[4.4]nonane-1,3-dione, a spirosuccinimide moiety of asperparaline A
- novel spiropentanopyrrolizidine oxime alkaloids, namely 2′,3′,5′,6′,7′,7a′-hexahydro-2,2-dimethylspirocyclopentane-1
- δ,δ-dimethyl-δ-valerolactone, via Baeyer-Villiger oxidation
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
91.4 °F - closed cup
Flash Point(C)
33 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Regioselective Synthesis of 2, 2-Dimethylcyclopentanone Using 2-Pyrrolidone Magnesium Salt as Electrogenerated Base.
Bonafoux D, et al.
Synthetic Communications, 28(1), 93-98 (1998)
Hiroshi Saito et al.
The Journal of steroid biochemistry and molecular biology, 136, 3-8 (2013-02-19)
Up to the present, numerous vitamin D derivatives have been synthesized, but most of them have straight side chains, and there are few publications described about in vitro and in vivo evaluations on bone by vitamin D derivatives. In our
S Tanimori et al.
Bioscience, biotechnology, and biochemistry, 64(8), 1758-1760 (2000-09-19)
2,6,6-Trimethyl-2-azaspiro[4.4]nonane-1,3-dione (9), a spirosuccinimide moiety of asperparaline A (1), was synthesized by starting from 2,2-dimethylcyclopentanone (4) via trinitrile 6 in five steps in a moderate yield. This conversion establishes a model study for synthesis of the spirosuccinimide moiety of asperparaline
Edwin Vedejs et al.
Journal of the American Chemical Society, 125(14), 4166-4173 (2003-04-03)
A new class of chiral phosphines belonging to the P-aryl-2-phosphabicyclo[3.3.0]octane family (PBO) has been prepared by enantioselective synthesis starting from lactate esters and 2,2-dimethylcyclopentanone enolate 5. A selective enolate alkylation method has been developed for preparation of 9 and 10
Synthesis of pyrrolizidine oximes 222 and 236: Novel alkaloids of a dendrobatid poison frog.
Rutchinson KD, et al.
Tetrahedron, 50(21), 6129-6136 (1994)
Articles
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service