323594
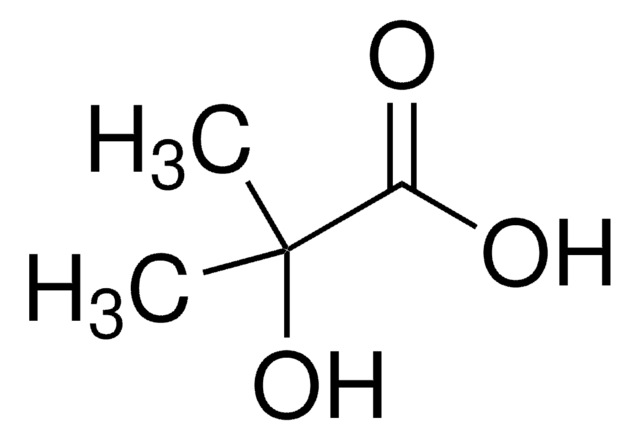
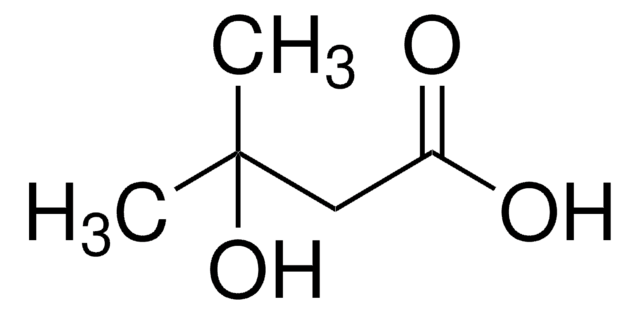
α-Hydroxyisobutyric acid
99%
Synonym(s):
alpha-Hydroxyisobutyric acid, 2-Hydroxy-2-methylpropionic acid, 2-Methyllactic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2C(OH)COOH
CAS Number:
Molecular Weight:
104.10
Beilstein:
1744739
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
84 °C/1.5 mmHg (lit.)
mp
76-80 °C (lit.)
functional group
carboxylic acid
hydroxyl
SMILES string
CC(C)(O)C(O)=O
InChI
1S/C4H8O3/c1-4(2,7)3(5)6/h7H,1-2H3,(H,5,6)
InChI key
BWLBGMIXKSTLSX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
α-Hydroxyisobutyric acid is a versatile building block in organic synthesis. It serves as a precursor in the synthesis of compounds like isobutylene glycol and methacrylic acid
Application
α-Hydroxyisobutyric acid (HIBA) can be used as:
- A chelating agent to improve the separation of lanthanides and actinides by liquid chromatography.
- In the synthesis of room temperature–stabilized, pure, nanocrystalline β-NiMoO4.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bimetallic single-source precursor for the synthesis of pure nanocrystalline room temperature-stabilized β-NiMoO4
Moneeb AM, et al.
Ceramics International, 42(1), 1366-1372 (2016)
Electrospray ionization mass spectrometric studies on uranyl complex with α-hydroxyisobutyric acid in water-methanol medium
Jaison PG, et al.
Rapid Communications in Mass Spectrometry, 27(10), 1105-1118 (2013)
Nadya Yaneva et al.
The Journal of biological chemistry, 287(19), 15502-15511 (2012-03-22)
Coenzyme B(12)-dependent acyl-CoA mutases are radical enzymes catalyzing reversible carbon skeleton rearrangements in carboxylic acids. Here, we describe 2-hydroxyisobutyryl-CoA mutase (HCM) found in the bacterium Aquincola tertiaricarbonis as a novel member of the mutase family. HCM specifically catalyzes the interconversion
A Amberg et al.
Toxicological sciences : an official journal of the Society of Toxicology, 51(1), 1-8 (1999-09-25)
Methyl-tert-butyl ether (MTBE) is widely used as an additive to gasoline to increase oxygen content and reduce tail pipe emission of pollutants. Therefore, widespread human exposure may occur. To contribute to the characterization of potential adverse effects of MTBE, its
Ana Carolina O Costa et al.
Journal of chromatography. A, 1171(1-2), 140-143 (2007-10-09)
The aim of this work was to develop a fast method using capillary electrophoresis for the determination of creatinine in human urine samples. The pH and constituents of the background electrolyte were selected by inspection of effective mobility of creatinine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service