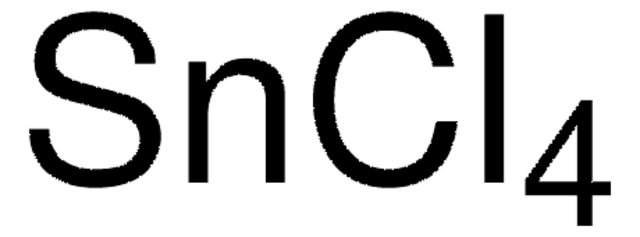307793
Titanium(IV) bromide
98%
Synonym(s):
Titanium tetrabromide
About This Item
Recommended Products
Quality Level
Assay
98%
form
solid
reaction suitability
core: titanium
reagent type: catalyst
bp
230 °C (lit.)
mp
38-40 °C (lit.)
density
3.25 g/mL at 25 °C (lit.)
SMILES string
Br[Ti](Br)(Br)Br
InChI
1S/4BrH.Ti/h4*1H;/q;;;;+4/p-4
InChI key
UBZYKBZMAMTNKW-UHFFFAOYSA-J
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- α-bromomethylene aldols by reacting with arylaldehydes and but-3-yn-2-one via Baylis-Hillman reaction.
- Titanium(II) bromide which along with copper forms an efficient catalytic system for Pinacol reaction.
- α-bromo-γ-hydroxyenamides by the reaction of ynamides and aldehyde or ketones.
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible, corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Titanium dioxide (TiO2) is an important n-type semiconducting material that shows interesting characteristics such as photoswitchable surface wettability, high photocatalytic activity, bistable electrical resistance states and high electron drift mobility.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service