303208
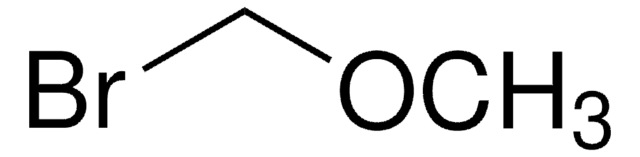
Bromomethyl acetate
95%
Synonym(s):
Acetoxymethyl bromide, Acetyloxymethyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3CO2CH2Br
CAS Number:
Molecular Weight:
152.97
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.447 (lit.)
bp
130-133 °C/750 mmHg (lit.)
density
1.56 g/mL at 25 °C (lit.)
functional group
bromo
ester
storage temp.
2-8°C
SMILES string
CC(=O)OCBr
InChI
1S/C3H5BrO2/c1-3(5)6-2-4/h2H2,1H3
InChI key
NHYXMAKLBXBVEO-UHFFFAOYSA-N
Related Categories
General description
The interaction of bromomethyl acetate with O(6)-alkylguanine-DNA alkyltransferase (DNA repair protein) was studied in vitro.
Application
Bromomethyl acetate was used in synthesis of optically active cyclohexene antisepsis agent, ethyl (6R)-6-[N-(2-chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate [(R)-1: TAK-242)]. It was also used as reagent in samarium diiodide-mediated conversion of ketones and aldehydes to 1,2-diacetates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
134.6 °F - closed cup
Flash Point(C)
57 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A direct preparation of 1, 2-diacetates from aldehydes and ketones promoted by samarium diiodide.
Enholm EJ and Satici H.
Tetrahedron Letters, 32(22), 2433-2436 (1991)
Masami Yamada et al.
Chemical & pharmaceutical bulletin, 54(1), 58-62 (2006-01-06)
Two new synthetic methods were established for the efficient synthesis of optically active cyclohexene antisepsis agent, ethyl (6R)-6-[N-(2-chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate [(R)-1: TAK-242)]. The first method involved recrystallization from methanol of the diastereomeric mixture (6RS,1'R)-7, obtained by esterification of carboxylic acid 3 with
Liping Liu et al.
Chemical research in toxicology, 17(6), 742-752 (2004-06-23)
O(6)-Alkylguanine-DNA alkyltransferase (AGT) is a DNA repair protein that removes O(6)-alkylguanine adducts. The interaction of dibromomethane (CH(2)Br(2)) and bromomethyl acetate (BrCH(2)OAc) with AGT was studied in vitro, and the effect of AGT on their toxicity and mutagenicity was investigated using
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service