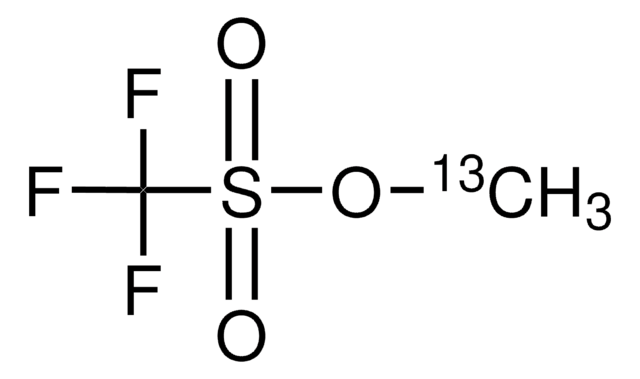
281077
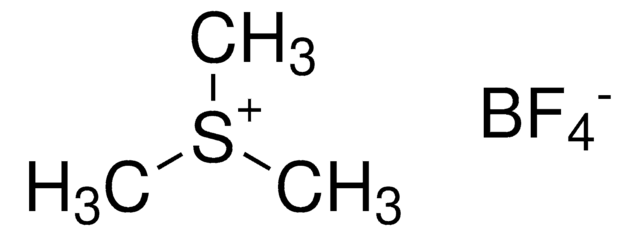
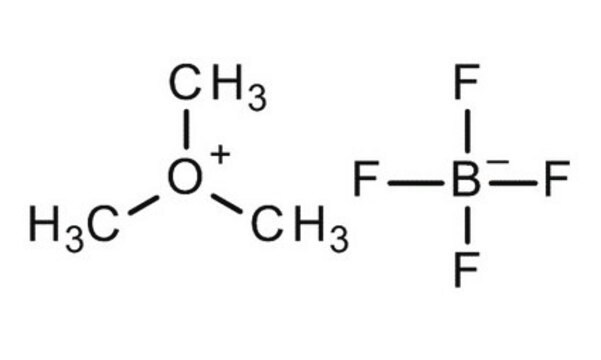
Trimethyloxonium tetrafluoroborate
95%
Synonym(s):
Trimethyloxonium fluoroborate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3O(BF4)
CAS Number:
Molecular Weight:
147.91
Beilstein:
3597303
EC Number:
MDL number:
UNSPSC Code:
12352107
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
storage temp.
−20°C
SMILES string
C[O+](C)C.F[B-](F)(F)F
InChI
1S/C3H9O.BF4/c1-4(2)3;2-1(3,4)5/h1-3H3;/q+1;-1
InChI key
CZVZBKHWOFJNCR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Trimethyloxonium tetrafluoroborate can be used as a methylating agent for the methylation of hydroxyl/carboxyl functional groups. It is capable of methylating polyfunctional carboxylic acids. It is also used as a catalyst for the polymerization of cyclic sulfides and ethers.
Application
Reagent for the methylation of hydroxyl groups recently used in a complex, multistep synthesis directed towards spirastrellolide, a marine natural product.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Toward the total synthesis of spirastrellolide A. Part 2: Conquest of the northern hemisphere.
Alois Fürstner et al.
Angewandte Chemie (International ed. in English), 45(33), 5510-5515 (2006-08-15)
H M Liebich et al.
Journal of chromatography. A, 843(1-2), 237-245 (1999-07-10)
Trimethyloxonium tetrafluoroborate (TMO) is applied as derivatising reagent to transform urinary organic acids into their methyl esters. The method is suggested as an alternative to the use of diazomethane which is carcinogenic and explosive. In contrast to other methods avoiding
Marco Pacenti et al.
Biomedical chromatography : BMC, 22(10), 1155-1163 (2008-05-29)
A method for the determination of the organic acids directly in the urine employing derivatization with trimethyloxonium tetrafluoroborate as a methylating agent and sequential extraction by head space and direct immersion/solid phase microextraction is reported. Furoic acid, hippuric acid, methylhippuric
S Chericoni et al.
Journal of analytical toxicology, 35(4), 193-198 (2011-04-26)
The present work describes the validation of a novel aqueous in situ derivatization procedure with trimethyloxonium tetrafluoroborate (TMO) as methylating agent for the simultaneous, quantitative analysis of Δ(9)-tetrahydrocannabinol (THC) and 11-nor-Δ(9)-tetrahydrocannabinol carboxylic acid (THC-COOH) in human urine. The derivatizing agent
H M Liebich et al.
Journal of chromatography. B, Biomedical sciences and applications, 713(2), 427-432 (1998-09-24)
We developed a new sample preparation method for profiling organic acids in urine by GC or GC-MS. The method includes derivatisation of the organic acids directly in the aqueous urine using trimethyloxonium tetrafluoroborate as a methylating agent, extraction of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service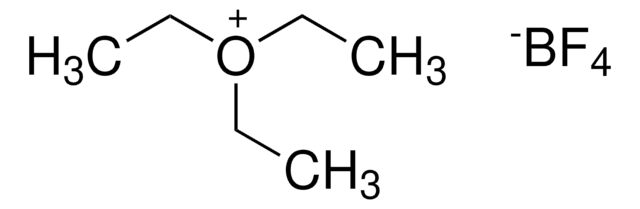

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)