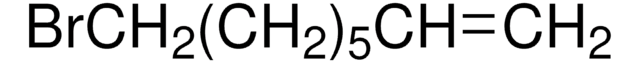253901
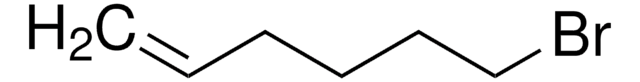
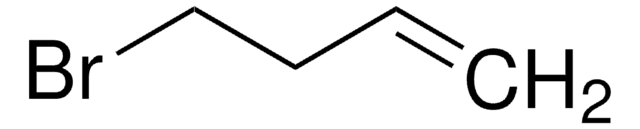
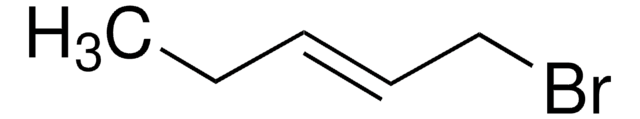
5-Bromo-1-pentene
95%
Synonym(s):
1-Bromo-4-pentene, 4-Pentenyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH2)3CH=CH2
CAS Number:
Molecular Weight:
149.03
Beilstein:
506077
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.463 (lit.)
bp
126-127 °C/765 mmHg (lit.)
density
1.258 g/mL at 25 °C (lit.)
functional group
alkyl halide
allyl
bromo
storage temp.
2-8°C
SMILES string
BrCCCC=C
InChI
1S/C5H9Br/c1-2-3-4-5-6/h2H,1,3-5H2
InChI key
LPNANKDXVBMDKE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
5-Bromo-1-pentene was used in stereoselective synthesis of 7α-(3-carboxypropyl) estradiol. It was used in preparation of thioacetate 11 of sialic acid having thioglycosidic linkage. It was also used as staring material in recent syntheses of DL-histrionicotoxin and benzophenone-containing fatty acids.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
87.8 °F - closed cup
Flash Point(C)
31 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jun-Ichi Sakamoto et al.
Bioorganic & medicinal chemistry letters, 17(3), 717-721 (2006-11-11)
An efficient synthesis of a series of carbosilane dendrimers uniformly functionalized with alpha-thioglycoside of sialic acid was accomplished. The results of a preliminary study on biological responses against influenza virus sialidases using thiosialoside clusters showed that some of the glycodendrimers
Yonghong Gan et al.
The Journal of organic chemistry, 71(25), 9487-9490 (2006-12-02)
Syntheses of new benzophenone-containing fatty acids (FABPs) 1, 5, and 6 and a new route to FABP 3 are described. Combined with the known 2 and 4, these FABPs comprise a set of photoactivatable fatty acid analogues with the crosslinking
M Adamczyk et al.
Steroids, 62(12), 771-775 (1998-01-22)
Alkylation of 3,17 beta-bis(2-trimethylsilyl)ethoxymethyl-1,3,5(10) estratriene-6-one (2) with 5-bromo-1-pentene using NaHMDS in THF afforded 3,17 beta-bis(2-trimethylsilyl)ethoxymethyl-7-alpha-(4'pentenyl)-1,3,5(10) estratriene-6-one (3) in excellent stereoselectivity (> 95% epimeric excess). Functionalization of the side chain in compound 3 was accomplished via ozonolysis, oxidation and esterification to
Maheswaran S Karatholuvhu et al.
Journal of the American Chemical Society, 128(39), 12656-12657 (2006-09-28)
The synthesis of (+/-)-histrionicotoxin has been achieved in just nine steps using a two-directional synthesis strategy. Key reactions include a two-directional cross-metathesis, a tandem oxime formation/Michael addition/1,4-prototopic shift/[3 + 2]-cycloaddition cascade, a selective Z,Z-bisenyne formation, and a one-pot N-O and
Maher A Qaddoura et al.
International journal of molecular sciences, 10(11), 4772-4788 (2010-01-21)
Several divinylic mesogenic monomers were synthesized based on coupling the monomer 4-(4-pentenyloxy)benzoic acid with chlorohydroquinone, 2,5-dihydroxy- acetophenone, methylhydroquinone or 2-methoxyhydroquinone. This resulted in novel mesogens of phenylene esters with different lateral substituent groups. The effect of the lateral substituent group
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 253901-25G | 4061825982434 |
| 253901-5G | 4061825982465 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service