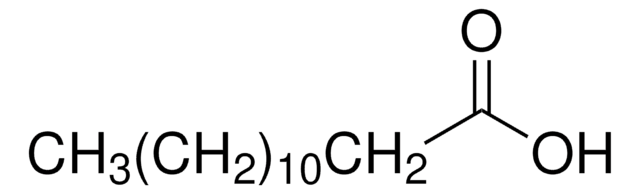All Photos(1)
About This Item
Linear Formula:
CH3(CH2)9COCl
CAS Number:
Molecular Weight:
204.74
Beilstein:
1759226
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
refractive index
n20/D 1.443 (lit.)
bp
135-136 °C/20 mmHg (lit.)
functional group
acyl chloride
SMILES string
CCCCCCCCCCC(Cl)=O
InChI
1S/C11H21ClO/c1-2-3-4-5-6-7-8-9-10-11(12)13/h2-10H2,1H3
InChI key
JUKPJGZUFHCZQI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Undecanoyl chloride has been used in the synthesis of:
- chrysotrione B, 2-acylcyclopentene-1,3-dione derivative, isolated from the fruiting bodies of the basidiomycete Hygrophorus chrysodon
- 2-methylpentadecan-5-one
- 4-ketotetradecanoic acid
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
71. Experiments on the synthesis of carbonyl compounds. Part II. A general synthesis of saturated ketones, R? CO? CH2R'.
Bowman RE.
Journal of the Chemical Society, 325-329 (1950)
Gianluca Gilardoni et al.
Journal of natural products, 70(1), 137-139 (2007-01-27)
Chrysotriones A (1) and B (2), two new 2-acylcyclopentene-1,3-dione derivatives, were isolated from the fruiting bodies of the Basidiomycete Hygrophorus chrysodon, and their structures were established by spectroscopic data and synthesis of compound 2. They represent the first examples of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service