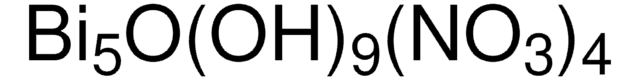248592
Bismuth(III) nitrate pentahydrate
reagent grade, 98%
Synonym(s):
Bismuth trinitrate pentahydrate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
Bi(NO3)3 · 5H2O
CAS Number:
Molecular Weight:
485.07
EC Number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
reagent grade
Quality Level
Assay
98%
form
crystals
reaction suitability
core: bismuth
reagent type: catalyst
bp
75-80 °C (lit.)
mp
30 °C (lit.)
SMILES string
O=[N+](O[Bi](O[N+]([O-])=O)O[N+]([O-])=O)[O-].C
InChI
1S/CH4.Bi.3NO3/c;;3*2-1(3)4/h1H4;;;;/q;+3;3*-1
InChI key
DPLOIMSBRDLLRI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Bismuth(III) nitrate pentahydrate is the hydrated form of bismuth(III) nitrate. It is effectively used as a Lewis acid catalyst in organic synthesis for the conversion of thiocarbonyls to their carbonyl compounds; aromatic nitration; protection of carbonyl compounds; Michael reactions, and synthesis of coumarins.
Application
An excellent oxidant for a variety of 4-substituted Hantzsch 1,4-dihydropyridines. Convenient reagent for selective oxidation of sufides to sulfoxides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Ox. Sol. 2
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mashraqui, S.H. Karnik, M.A.
Synthesis, 713-713 (1998)
Mashraqui, S.H. et al.
Synthetic Communications, 28, 939-939 (1998)
Na Li et al.
Dalton transactions (Cambridge, England : 2003), 43(36), 13742-13750 (2014-08-12)
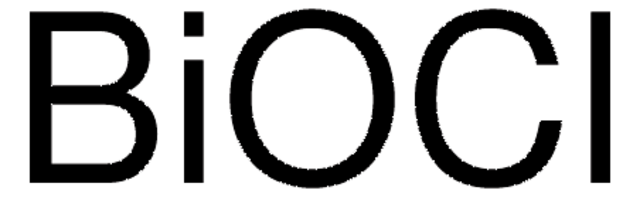
The xFe2O3/yBiOCl composites (xFe/yBi, x/y = 0/100, 5/100, 10/100, 20/100, 30/100 and 40/100 molar ratios) are prepared for the first time through an in situ hydrolysis method under hydrothermal conditions. The samples are characterized by X-ray diffraction (XRD), scanning electron
Nahid Gharib Naseri et al.
Analytical and bioanalytical chemistry, 391(4), 1283-1292 (2008-03-21)
Catalytic adsorptive stripping voltammetry (CAdSV) has been demonstrated at a bismuth film electrode (BiFE) in an injection-moulded electrochemical micro-flow cell. The polystyrene three-electrode flow cell was fabricated with electrodes moulded from a conducting grade of polystyrene containing 40% carbon fibre
Marcela Luísa Gomes et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 20(5), 771-779 (2015-05-02)
Two bismuth(III) porphyrins-5,10,15,20-tetrakis(phenyl)porphyrinatobismuth(III) nitrate, [Bi(III)(TPP)]NO3, and the unprecedent 5,10,15,20-tetrakis(4-carbomethoxyphenyl)porphyrinatobismuth(III) nitrate, [Bi(III)(T4CMPP)]NO3, and two unprecedented antimony(V) porphyrins dichlorido(5,10,15,20-tetrakis(phenyl)porphyrinato)antimony(V) bromide, [Sb(V)(TPP)Cl2]Br, and dibromido(5,10,15,20-tetrakis(4-carbomethoxyphenyl)porphyrinato)antimony(V) bromide, [Sb(V)(T4CMPP)Br2]Br,-were synthesized by reacting the corresponding porphyrin ligand with Bi(NO3)3·5H2O or SbCl3. All compounds were characterized by UV-vis
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 248592-500G | 4061838252708 |
| 248592-100G | 4061825928234 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service