238864
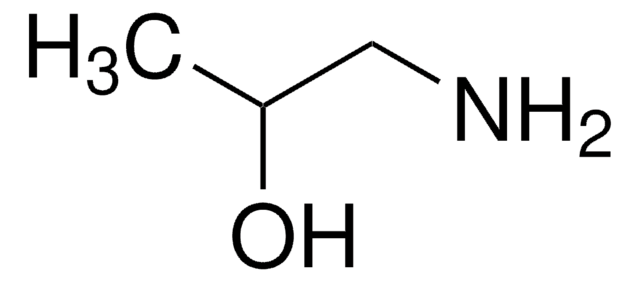
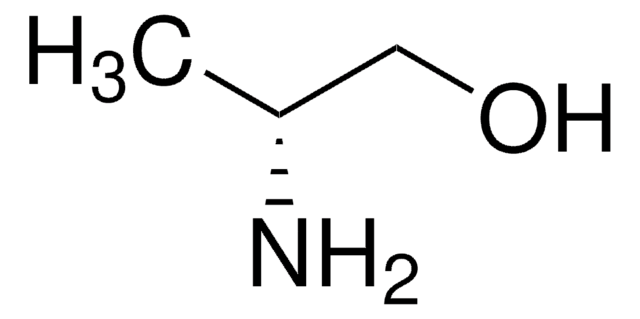
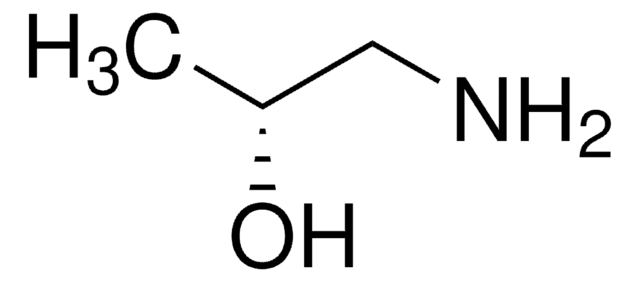
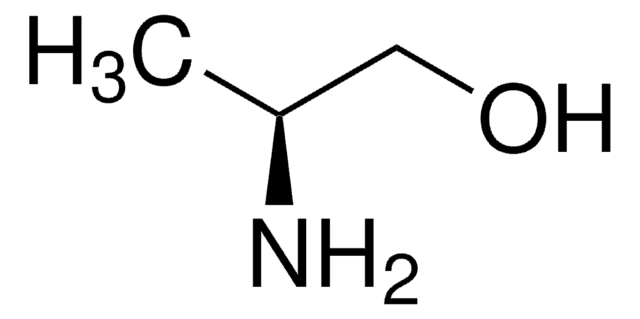
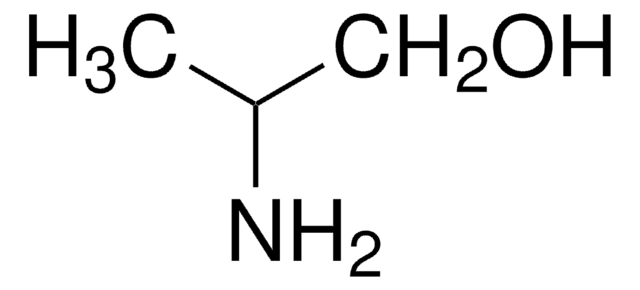
(S)-(+)-1-Amino-2-propanol
97%
Synonym(s):
(+)-Isopropanolamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH(OH)CH2NH2
CAS Number:
Molecular Weight:
75.11
Beilstein:
1718868
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
2.6 (vs air)
Quality Level
vapor pressure
<1 mmHg ( 20 °C)
Assay
97%
optical activity
[α]20/D +18°, c = 1.8 in H2O
refractive index
n20/D 1.4437 (lit.)
bp
160 °C (lit.)
mp
24-26 °C (lit.)
density
0.954 g/mL at 25 °C (lit.)
functional group
amine
hydroxyl
SMILES string
C[C@H](O)CN
InChI
1S/C3H9NO/c1-3(5)2-4/h3,5H,2,4H2,1H3/t3-/m0/s1
InChI key
HXKKHQJGJAFBHI-VKHMYHEASA-N
Looking for similar products? Visit Product Comparison Guide
General description
(S)-(+)-1-Amino-2-propanol is a chiral amino alcohol.
Application
(S)-(+)-1-Amino-2-propanol may be used in the preparation of S-(+)-1-(2,3-naphthalimido)-2-propanol.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
159.8 °F - closed cup
Flash Point(C)
71 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Highly Enantioselective asymmetric hydrogenation of a-phthalimide ketone: an efficient entry to enantiomerically pure amino alcohols.
Lei A, et al.
Journal of the American Chemical Society, 126(6), 1626-1627 (2004)
S-(+)-1-Methyl-2-(2, 3-naphthalimido) ethyl Trifluoromethanesulfonate as a Fluorescence Chiral Labeling Reagent for Carboxylic Acid Enantiomers.
Yasaka Y, et al.
Analytical Sciences, 11(2), 295-297 (1995)
D Catone et al.
The Journal of chemical physics, 127(14), 144312-144312 (2007-10-16)
Valence band and C 1s core-level photoelectron spectra of S-(+)-2-amino-1-propanol (alaninol) and S-(+)-1-amino-2-propanol (isopropanolamine) have been studied by means of synchrotron radiation photoelectron spectroscopy in gas phase. The alaninol, the reduced derivative of the alanine, is a good test system
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service