220108
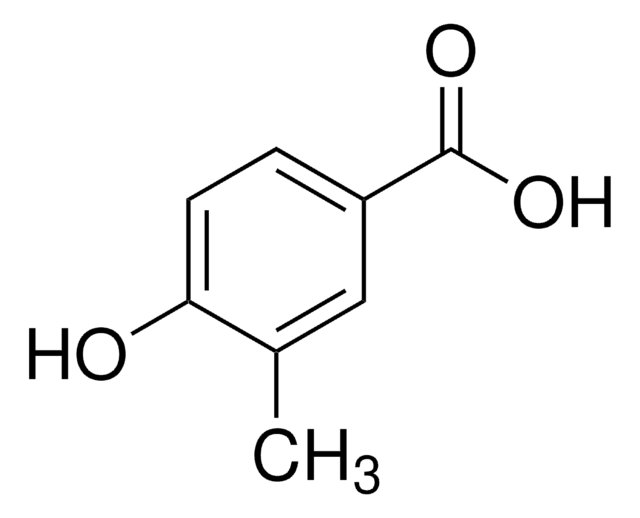
Isovanillic acid
97%
Synonym(s):
3-Hydroxy-4-methoxybenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H3(OCH3)CO2H
CAS Number:
Molecular Weight:
168.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
250-253 °C (lit.)
functional group
carboxylic acid
SMILES string
COc1ccc(cc1O)C(O)=O
InChI
1S/C8H8O4/c1-12-7-3-2-5(8(10)11)4-6(7)9/h2-4,9H,1H3,(H,10,11)
InChI key
LBKFGYZQBSGRHY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Rate of oxidation of isovanillic acid by washed cells of Pseudomonas putida has been reported.
Application
Isovanillic acid has been used in the synthesis of opiate alkaloid precursor, dihydrothebainone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Widiastuti Setyaningsih et al.
Food chemistry, 288, 221-227 (2019-03-25)
Fifteen phenolic compounds were determined in rice grains by ultrasound assisted extraction and ultra-performance liquid chromatography with photodiode array detection. Primarily, an UAE method has been developed and validated for the extraction of phenolics from rice grains. For the optimization
Tomas Strucko et al.
Scientific reports, 7, 41431-41431 (2017-01-31)
Over-expression of a gene by increasing its copy number is often desirable in the model yeast Saccharomyces cerevisiae. It may facilitate elucidation of enzyme functions, and in cell factory design it is used to increase production of proteins and metabolites.
Preparation of oxygenated phenylacetic acids.
Weller DD, et al.
The Journal of Organic Chemistry, 49(11), 2061-2063 (1984)
Zhuxing Tang et al.
Journal of pharmaceutical and biomedical analysis, 40(2), 484-489 (2005-09-17)
A method based on capillary electrophoresis with electrochemical detection has been developed for the separation and determination of isovanillic acid, gentisic acid, kaempferol, quercetin, caffeic acid and protocatechuic acid in Ilex Purpurea Hassk and its medicinal preparations for the first
M A Fernández et al.
Journal of ethnopharmacology, 53(1), 11-14 (1996-07-26)
The phenolic fractions of aerial part of Scrophularia frutescens and S. sambucifolia (Scrophulariaceae) showed a potent antibacterial activity. Ferulic, isovanillic, p-hydroxycinnamic, p-hydroxybenzoic, syringic, caffeic, gentisic and protocatechuic acids were isolated from S. frutescens and ferulic, p-coumaric, vanillic, p-hydroxibenzoic and syringic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service