19390
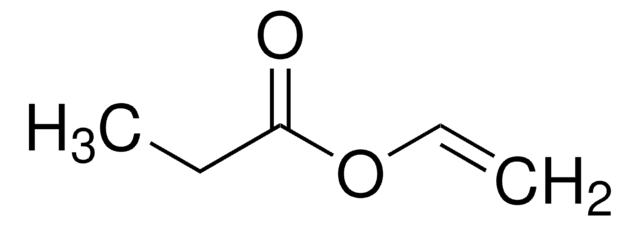
Vinyl butyrate
contains 20 ppm 4-methoxyphenol as stabilizer, ≥99.0% (GC)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH2CH2COOCH=CH2
CAS Number:
Molecular Weight:
114.14
Beilstein:
1744933
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (GC)
contains
20 ppm 4-methoxyphenol as stabilizer
refractive index
n20/D 1.410
density
0.899 g/mL at 20 °C
functional group
ester
storage temp.
2-8°C
SMILES string
CCCC(=O)OC=C
InChI
1S/C6H10O2/c1-3-5-6(7)8-4-2/h4H,2-3,5H2,1H3
InChI key
MEGHWIAOTJPCHQ-UHFFFAOYSA-N
Related Categories
General description
Vinyl butyrate causes the transesterification of natural flavonoid bergenin immobilized onto carboxylic acid functionalized controlled pore glass.
Application
Vinyl butyrate was used as acyl donor in the synthesis of citronellyl esters (acetate, propionate, butyrate, caprate and laurate) using immobilized Candida antarctical ipase B.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
68.0 °F - closed cup
Flash Point(C)
20 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Giulia Martelli et al.
Bioorganic chemistry, 88, 102975-102975 (2019-05-19)
Obtainment and testing of pure enantiomers are of great importance for bioactive compounds, because of the assessed implications of enantioselectivity in receptor-mediated responses. Herein we evaluated the use of biocatalysis to obtain enantiomerically pure β-lactam intermediates further exploited in the
Betzaida Castillo et al.
Biotechnology and bioengineering, 94(3), 565-574 (2006-02-24)
Enzymes are attractive catalysts for the production of optically active compounds in organic solvents. However, their often low catalytic activity in such applications hampers their practical use. To overcome this, we investigated the effectiveness of the covalent modification of alpha-chymotrypsin
Ionic liquids improve citronellyl ester synthesis catalyzed by immobilized Candida antarctica lipase B in solvent-free media.
Lozano P, et al.
Green Chemistry, 9(7), 780-784 (2007)
Betzaida Castillo et al.
BMC biotechnology, 6, 51-51 (2006-12-26)
Enzymes have been extensively used in organic solvents to catalyze a variety of transformations of biological and industrial significance. It has been generally accepted that in dry aprotic organic solvents, enzymes are kinetically trapped in their conformation due to the
Mozaffar Shakeri et al.
Journal of biotechnology, 145(3), 281-283 (2009-12-02)
We examined the activity of free Rhizopus oryzae lipase (ROL) and ROL immobilized on mesoporous materials in transesterification reactions in various dialkylimidazolium-cation based ionic liquids. For free ROL, the highest activity (0.39 U/mg protein) was obtained in [OMIm][PF(6)] followed by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service