172472
Isopropyl myristate
98%
Synonym(s):
Isopropyl tetradecanoate, Myristic acid isopropyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
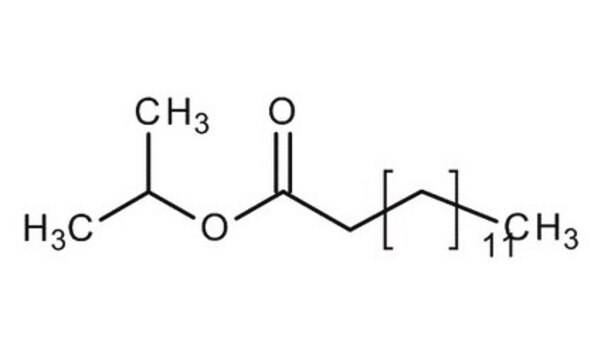
CH3(CH2)12COOCH(CH3)2
CAS Number:
Molecular Weight:
270.45
Beilstein:
1781127
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39022421
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.434 (lit.)
bp
193 °C/20 mmHg (lit.)
mp
~3 °C (lit.)
density
0.85 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCCCCC(=O)OC(C)C
InChI
1S/C17H34O2/c1-4-5-6-7-8-9-10-11-12-13-14-15-17(18)19-16(2)3/h16H,4-15H2,1-3H3
InChI key
AXISYYRBXTVTFY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
IPM is a fatty acid ester that can be produced by the esterification of isopropyl alcohol and myristic acid.
Application
Isopropyl myristate was used as oil phase in the preparation of formulations. It was also used as oil/water-type tacrolimus (FK506) solvent.
Storage Class Code
10 - Combustible liquids
WGK
awg
Flash Point(F)
>302.0 °F
Flash Point(C)
> 150 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Timothy S McClintock et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 34(47), 15669-15678 (2014-11-21)
Our understanding of mammalian olfactory coding has been impeded by the paucity of information about the odorant receptors (ORs) that respond to a given odorant ligand in awake, freely behaving animals. Identifying the ORs that respond in vivo to a
Neslihan Ustündağ Okur et al.
Chemical & pharmaceutical bulletin, 62(2), 135-143 (2014-02-05)
Naproxen (Np) is an example of a non-steroidal anti-inflammatory drug (NSAID) commonly used for the reduction of pain and inflammation. In order to develop an alternative formulation for the topical administration of Np, microemulsions were evaluated as delivery vehicles. Four
Masayuki Yamanaka et al.
International journal of pharmaceutics, 464(1-2), 19-26 (2014-01-25)
We developed an oil/water-type tacrolimus (FK506) cream formulation as an alternative to Protopic ointment for atopic dermatitis treatment. We determined the effects of solvents used in topical preparations on FK506 solubility and stability, and evaluated FK506 transdermal absorption into rat
Ji Zhang et al.
International journal of pharmaceutics, 421(1), 34-44 (2011-10-01)
In this study, microemulsion microstructures, key formulation variables, and their relationship to drug transdermal permeation enhancement were investigated. A microemulsion system with high water soluble capacity was developed, using isopropyl myristate, Labrasol, and Cremophor EL as oil, surfactant, and co-surfactant
Qingping Tian et al.
International journal of pharmaceutics, 426(1-2), 202-210 (2012-01-26)
To improve the skin permeation of naproxen with larger dosage, microemulsion with high content of naproxen was investigated for transdermal delivery and its solubilization mechanism was studied. Naproxen micoremulsions composed of 4% isopropyl myristate, 18% Tween 80, 18% ethanol and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service