165336
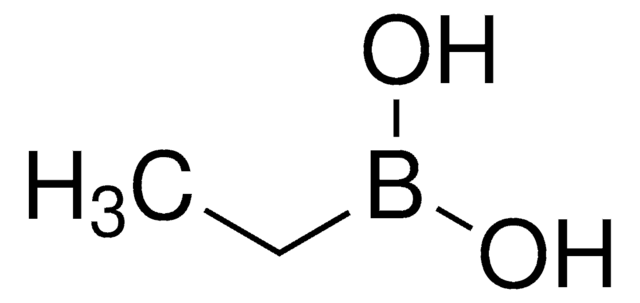
Methylboronic acid
97%
Synonym(s):
Methaneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3B(OH)2
CAS Number:
Molecular Weight:
59.86
Beilstein:
1731087
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
91-94 °C (lit.)
SMILES string
CB(O)O
InChI
1S/CH5BO2/c1-2(3)4/h3-4H,1H3
InChI key
KTMKRRPZPWUYKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Methylboronic acid can be used as a reagent:
- In the palladium-catalyzed Stille and Suzuki-Miyaura cross-couplings.
- In the microwave-heated heterogeneous palladium (Pd)-catalytized reactions.
- In ruthenium (Ru)-catalyzed silylation reactions
- To prepare bis(aminotropone) titanium (Ti) catalysts for ethylene polymerizations.
- In the enantioselective asymmetric bromoaminocyclization and bromoaminocyclization using amino-thiocarbamate catalysts.
- To prepare common building blocks for pharmaceuticals and agrochemicals.
- To prepare chrysin analogs by Suzuki-Miyaura coupling reactions.
- To prepare casein kinase I inhibitors.
- In the divergent C-H functionalizations directed by sulfonamide pharmacophores in drug discovery.
- In the synthesis of unsymmetrical monosulfides from disulfides via copper-catalyzed coupling with boronic acids.
- In a palladium-catalyzed coupling with enol tosylates.
- For derivatizing many carbohydrates and biologically active compounds for GLC analysis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dietrich Steinhuebel et al.
The Journal of organic chemistry, 70(24), 10124-10127 (2005-11-19)
[reaction: see text] Herein we demonstrate functionalized enol tosylates to be robust substrates that undergo Suzuki-Miyaura, Sonogashira, and Stille cross-coupling reactions to provide stereodefined trisubstituted unsaturated esters.
Ben W Glasspoole et al.
Chemical communications (Cambridge, England), 48(9), 1230-1232 (2011-12-20)
Palladium-catalyzed cross-coupling reactions of secondary allylic boronic esters with iodoarenes were demonstrated under the conditions previously described for the coupling of benzylic substrates. The regioselectivity of the process was largely dictated by the pattern of olefin substitution.
A comparative study of ethylene polymerization by bis(aminotropone) Ti catalysts
Goldani, M. T.; et al.
Polym. Bull., 68, 755-773 (2012)
Ling Zhou et al.
Journal of the American Chemical Society, 132(44), 15474-15476 (2010-10-16)
A novel amino-thiocarbamate-catalyzed bromolactonization of unsaturated carboxylic acids has been developed. The scope of the reaction is evidenced by 22 examples of γ-lactones with up to 99% yield and 93% ee. The protocol was applied in the enantioselective synthesis of
Ling Zhou et al.
Journal of the American Chemical Society, 133(24), 9164-9167 (2011-05-05)
A facile and efficient enantioselective bromoaminocyclization of unsaturated sulfonamides has been developed using an amino-thiocarbamate catalyst. A range of enantioenriched pyrrolidines were prepared with up to 99% yield and 99% ee. The corresponding lactams could be obtained through oxidation of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service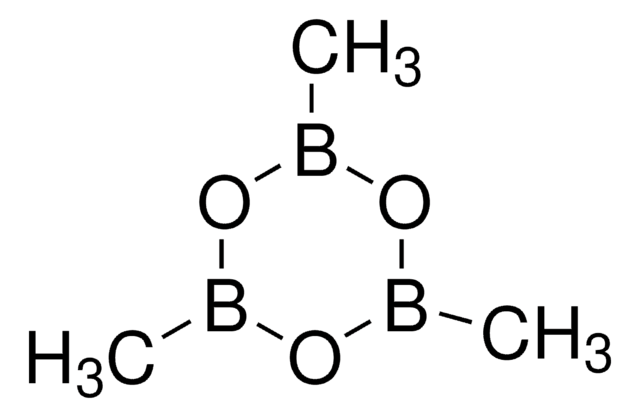

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)