161144
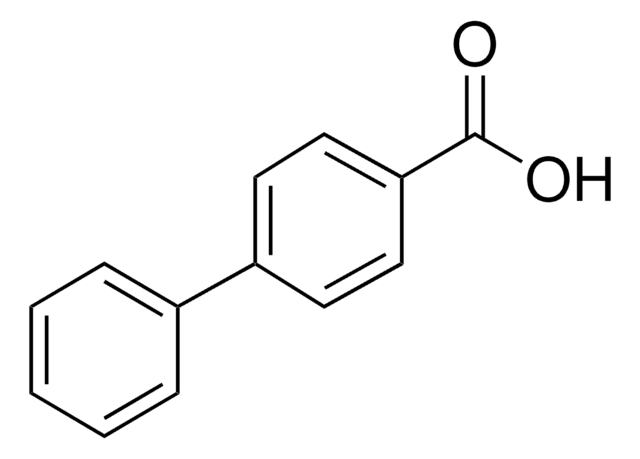
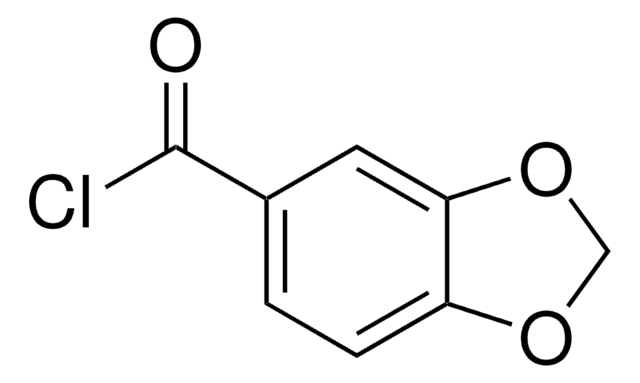
Biphenyl-4-carbonyl chloride
97%
Synonym(s):
4-Phenylbenzoyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5C6H4COCl
CAS Number:
Molecular Weight:
216.66
Beilstein:
472842
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
110-112 °C (lit.)
functional group
acyl chloride
phenyl
SMILES string
ClC(=O)c1ccc(cc1)-c2ccccc2
InChI
1S/C13H9ClO/c14-13(15)12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9H
InChI key
JPVUWCPKMYXOKW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Biphenyl-4-carbonyl chloride was used in the preparation of a novel thiourea compound, N-(6-methyl pyridin-2-yl-carbamothioyl)biphenyl-4-carboxamide. It was also used in the preparation of 5-CF3-oxazole analog, 2-{4-[2-(2-biphenyl-4-yl-5-trifluoromethyl-oxazol-4-yl)-ethoxy]-phenoxy}-2-methyl-propionic acid.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alexander G Godfrey et al.
The Journal of organic chemistry, 68(7), 2623-2632 (2003-03-29)
An improved method for the preparation of a series of oxazole-containing dual PPARalpha/gamma agonists is described. A synthetic sequence utilizing a Dakin-West reaction was devised that allows for the introduction of the oxazole ring either late in the synthetic sequence
Theoretical and experimental studies on N-(6-methylpyridin-2-yl-carbamothioyl) biphenyl-4-carboxamide.
Yesilkaynak T, et al.
European Journal of Chemistry, 1(1), 1-5 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service