154571
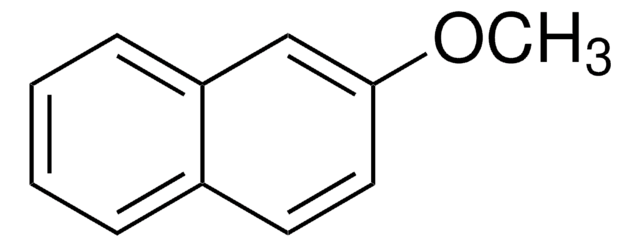
1-Methoxynaphthalene
≥98%
Synonym(s):
Methyl 1-naphthyl ether, NSC 5530
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C10H7OCH3
CAS Number:
Molecular Weight:
158.20
Beilstein:
774884
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
liquid
refractive index
n20/D 1.621 (lit.)
bp
135-137 °C/12 mmHg (lit.)
density
1.09 g/mL at 25 °C (lit.)
SMILES string
COc1cccc2ccccc12
InChI
1S/C11H10O/c1-12-11-8-4-6-9-5-2-3-7-10(9)11/h2-8H,1H3
InChI key
NQMUGNMMFTYOHK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-Methoxynaphthalene was used to study the peroxygenase activity of CcP. It was also used to synthesize prenyl naphthalen-ols.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
James E Erman et al.
BMC biochemistry, 14, 19-19 (2013-07-31)
The cytochrome P450s are monooxygenases that insert oxygen functionalities into a wide variety of organic substrates with high selectivity. There is interest in developing efficient catalysts based on the "peroxide shunt" pathway in the cytochrome P450s, which uses H2O2 in
Kazutoshi Shindo et al.
Bioscience, biotechnology, and biochemistry, 75(3), 505-510 (2011-03-11)
We performed combinational bioconversion of substituted naphthalenes with PhnA1A2A3A4 (an aromatic dihydroxylating dioxygenase from marine bacterium Cycloclasticus sp. strain A5) and prenyltransferase NphB (geranyltransferase from Streptomyces sp. strain CL190) or SCO7190 (dimethylallyltransferase from Streptomyces coelicolor A3(2)) to produce prenyl naphthalen-ols.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service