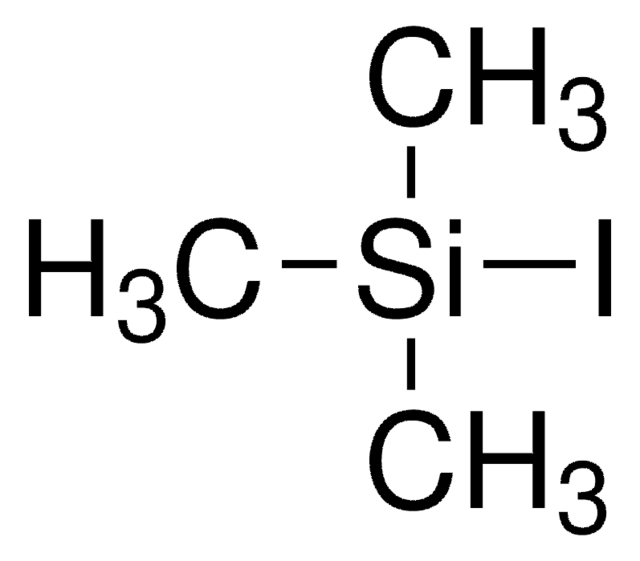153583
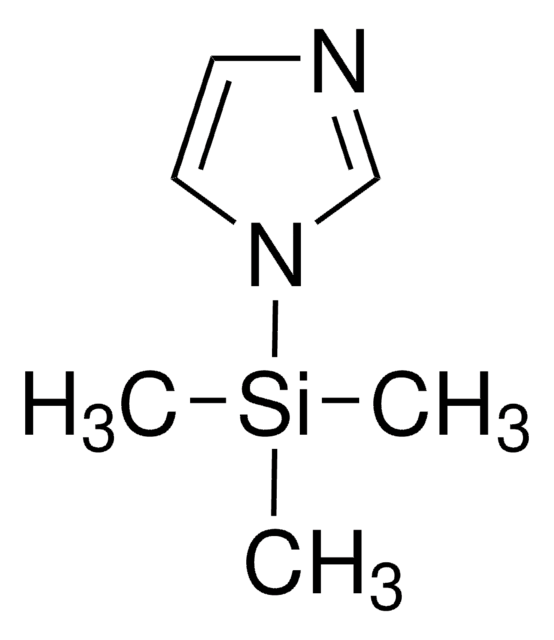
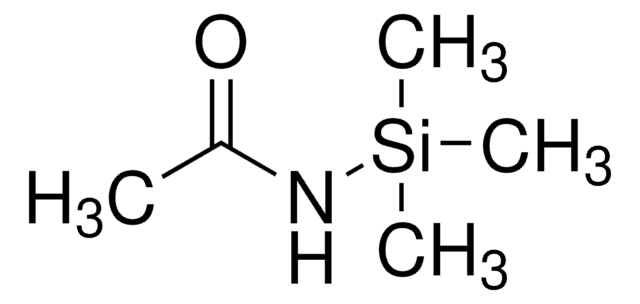
1-(Trimethylsilyl)imidazole
96%
Synonym(s):
N-Trimethylsilylimidazole, TMSI, TSIM
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H12N2Si
CAS Number:
Molecular Weight:
140.26
Beilstein:
606148
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
synthesis grade
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.475 (lit.)
bp
93-94 °C/14 mmHg (lit.)
density
0.956 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C[Si](C)(C)n1ccnc1
InChI
1S/C6H12N2Si/c1-9(2,3)8-5-4-7-6-8/h4-6H,1-3H3
InChI key
YKFRUJSEPGHZFJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-(Trimethylsilyl)imidazole (TMSI) was used for derivatization of carbohydrates into trimethylsilyl ethers. It was also used to synthesize polysubstituted chiral spirotetrahydropyrans and as silylating reagent for the protection of hydroxyl groups in the presence of amine functionalities.
Silylating reagent for the protection of hydroxyl groups in the presence of amine functionalities.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
42.8 °F - closed cup
Flash Point(C)
6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthetic Communications, 23, 2191-2191 (1993)
Marijana M Ačanski et al.
Food chemistry, 145, 743-748 (2013-10-17)
Gas chromatography with mass spectrometry was used for carrying out a qualitative analysis of the ethanol soluble flour extract of different types of cereals bread wheat and spelt and pseudocereals (amaranth and buckwheat). TMSI (trimethylsilylimidazole) was used as a reagent
Maiwenn Jacolot et al.
Organic letters, 14(1), 58-61 (2011-12-02)
We report a TMSI-promoted Prins cyclization reaction with ketones as carbonyl partners to prepare polysubstituted chiral spirotetrahydropyrans. In the presence of racemic 2-methylcyclohexanone a dynamic kinetic resolution occurred affording one stereoisomer. The observed enantiospecificity has been rationalized by DFT calculation.
Silylated N,O-ketals from the reaction of ketones with N-trimethylsilylimidazole.
P A Andrews et al.
Journal of chromatography, 419, 271-274 (1987-08-07)
Qin Zhao et al.
Journal of chromatography. A, 1240, 45-51 (2012-04-24)
In the present work, we developed a novel dispersive microextraction technique by combining the advantages of liquid-phase microextraction (LPME) and magnetic solid-phase extraction (MSPE). In this method, trace amount of water directly absorbed on bare Fe₃O₄ to form water-coated Fe₃O₄
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service