All Photos(2)
About This Item
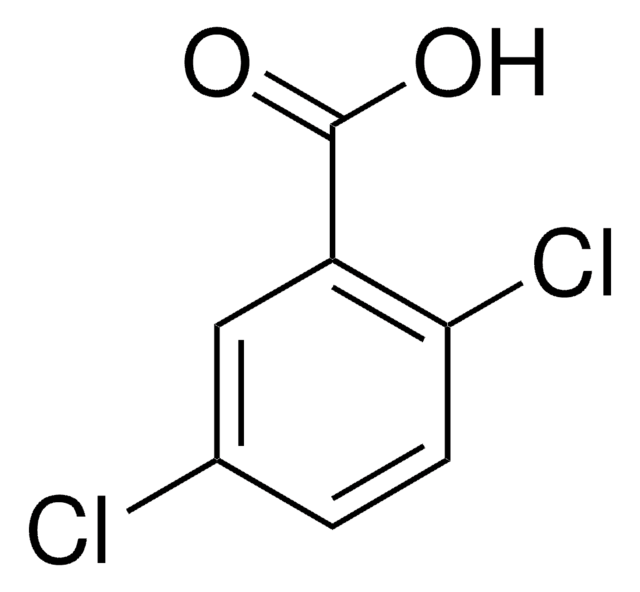
Linear Formula:
Cl2C6H3CO2H
CAS Number:
Molecular Weight:
191.01
Beilstein:
2044777
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
204-206 °C (lit.)
functional group
carboxylic acid
chloro
SMILES string
OC(=O)c1ccc(Cl)c(Cl)c1
InChI
1S/C7H4Cl2O2/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3H,(H,10,11)
InChI key
VPHHJAOJUJHJKD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,4-Dichlorobenzoic acid was employed as internal standard during the multiresidue analysis of pharmaceuticals and personal care products by ultra performance liquid chromatography-positive/negative electrospray tandem mass spectrometry. It was used to study the metabolic fate of 4-chloro-3,5-dinitrobenzoic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fate of substituted benzoates in the freshwater green alga, Chlamydomonas reinhardtii 11-32b.
Gutenkauf A, et al.
Biodegradation, 9(5), 359-368 (1998)
Barbara Kasprzyk-Hordern et al.
Analytical and bioanalytical chemistry, 391(4), 1293-1308 (2008-02-07)
The main aim of the presented research is to introduce a new technique, ultra performance liquid chromatography-positive/negative electrospray tandem mass spectrometry (UPLC-ESI/MS/MS), for the development of new simultaneous multiresidue methods (over 50 compounds). These methods were used for the determination
K Umehara et al.
Drug metabolism and disposition: the biological fate of chemicals, 28(8), 887-894 (2000-07-20)
The metabolism of 1-(3,4-dichlorobenzyl)-5-octylbiguanide (OPB-2045), a new potent biguanide antiseptic, was investigated using rat and dog liver preparations to elucidate the mechanism of OPB-2045 metabolite formation, in which the octyl side chain is reduced to four, five, or six carbon
P Adriaens et al.
Applied and environmental microbiology, 57(1), 173-179 (1991-01-01)
When Acinetobacter sp. strain 4-CB1 was grown on 4-chlorobenzoate (4-CB), it cometabolized 3,4-dichlorobenzoate (3,4-DCB) to 3-chloro-4-hydroxybenzoate (3-C-4-OHB), which could be used as a growth substrate. No cometabolism of 3,4-DCB was observed when Acinetobacter sp. strain 4-CB1 was grown on benzoate.
Yoshiteru Noutoshi et al.
Scientific reports, 2, 705-705 (2012-10-11)
Plant activators are agrochemicals that protect crops from pathogens. They confer durable resistance to a broad range of diseases by activating intrinsic immune mechanisms in plants. To obtain leads regarding useful compounds, we have screened a chemical library using an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service