All Photos(1)
About This Item
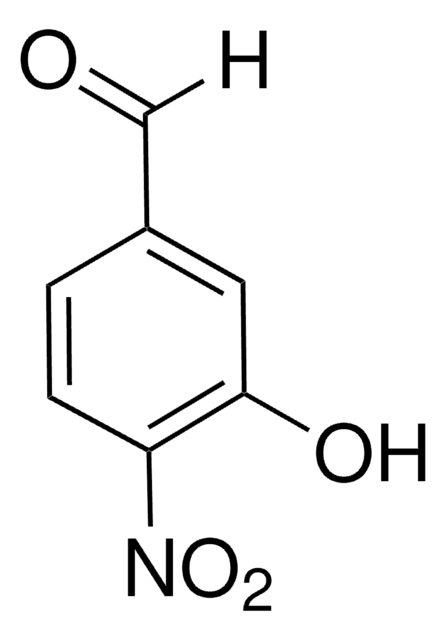
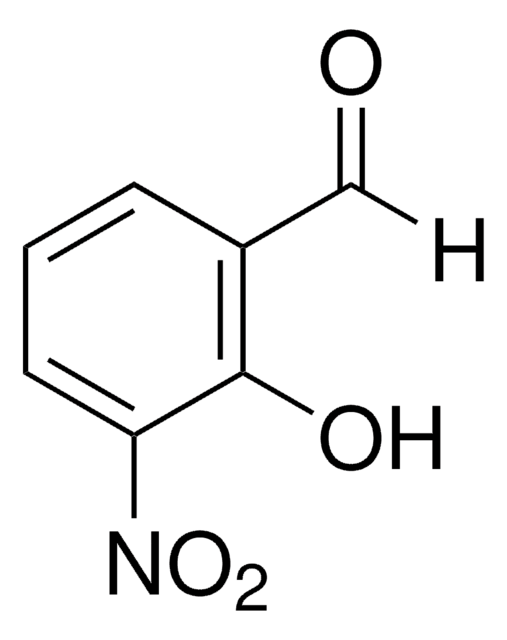
Linear Formula:
HOC6H3(NO2)CHO
CAS Number:
Molecular Weight:
167.12
Beilstein:
2047884
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
140-142 °C (lit.)
functional group
aldehyde
nitro
SMILES string
[H]C(=O)c1ccc(O)c(c1)[N+]([O-])=O
InChI
1S/C7H5NO4/c9-4-5-1-2-7(10)6(3-5)8(11)12/h1-4,10H
InChI key
YTHJCZRFJGXPTL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Hydroxy-3-nitrobenzaldehyde reacts with 3-bromobenzohydrazide in methanol to yield (E)-3-bromo-N′-(4-hydroxy-3-nitrobenzylidene)benzohydrazide.
Application
4-Hydroxy-3-nitrobenzaldehyde was used in the synthesis of:
- 4-hydroxy-3-nitrocinnamic acid
- solvated benzohydrazone derivatives: N′-(4-hydroxy-3-nitrobenzylidene)-3-methylbenzohydrazide-methanol-water
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yasuko Sakihama et al.
FEBS letters, 553(3), 377-380 (2003-10-24)
Peroxynitrite (ONOO(-)), a reactive nitrogen species, is capable of nitrating tyrosine residue of proteins. Here we show in vitro evidence that plant phenolic compounds can also be nitrated by an ONOO(-)-independent mechanism. In the presence of NaNO(2), H(2)O(2), and horseradish
Characterization and crystal structures of solvated N'-(4-hydroxy-3-nitrobenzylidene)-3-methylbenzohydrazide and N'-(4-dimethyl-aminobenzylidene)-3-methylbenzohydrazide.
Ma, J-J.
Journal of Structural Chemistry, 54(6), 1145-1150 (2013)
Guo-Biao Cao et al.
Acta crystallographica. Section E, Structure reports online, 65(Pt 8), o1725-o1725 (2009-01-01)
The title compound, C(14)H(10)BrN(3)O(4), was synthesized by the reaction of 4-hydr-oxy-3-nitro-benzaldehyde with an equimolar quantity of 3-bromo-benzohydrazide in methanol. The mol-ecule displays an E configuration about the C=N bond. The dihedral angle between the two benzene rings is 4.6 (2)°. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service