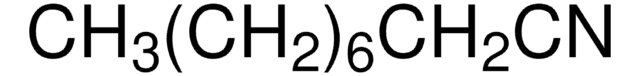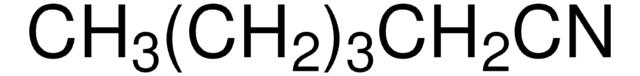138053
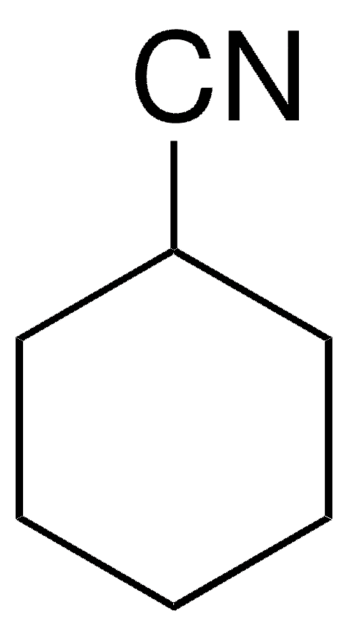
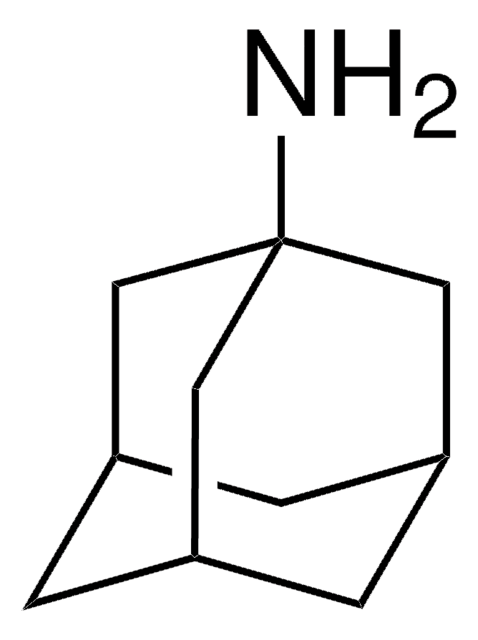
1-Adamantanecarbonitrile
97%
Synonym(s):
1-Cyanoadamantane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H15N
CAS Number:
Molecular Weight:
161.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
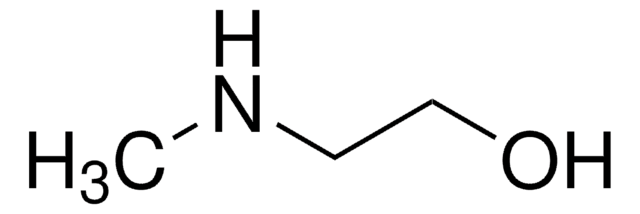
Recommended Products
Quality Level
Assay
97%
mp
193-196 °C (lit.)
SMILES string
N#CC12CC3CC(CC(C3)C1)C2
InChI
1S/C11H15N/c12-7-11-4-8-1-9(5-11)3-10(2-8)6-11/h8-10H,1-6H2/t8-,9+,10-,11-
InChI key
FQFZASRJFRAEIH-BIBSGERRSA-N
General description
1-Adamantanecarbonitrile reacts with W2(OCMe3)6 to yield W(CAd)(OCMe3)3 (Ad=1-adamantyl).
Application
1-Adamantanecarbonitrile was used as starting reagent for the synthesis of adamantyl-l,3,4-oxathiazol-2-one.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
α-Hydrogen migration reactions in tungsten (VI) cyclopentadienyl alkylidyne complexes.
Warren TH, et al.
Journal of Organometallic Chemistry, 569(1), 125-137 (1998)
The selective complexation of adamantane nitriles by tungsten pentacarbonyl.
Jefford VJ, et al.
Canadian Journal of Chemistry, 74(1), 107-113 (1996)
M Flämig et al.
The Journal of chemical physics, 151(22), 224507-224507 (2019-12-16)
The dynamics of cyanoadamantane (CN-ADA) in its plastically crystalline phase encompasses three processes: overall tumbling of the rigid molecule, rotation around the molecular symmetry axis, and vacancy diffusion. This makes CN-ADA a prototypical case to be studied by field-cycling as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service