134848
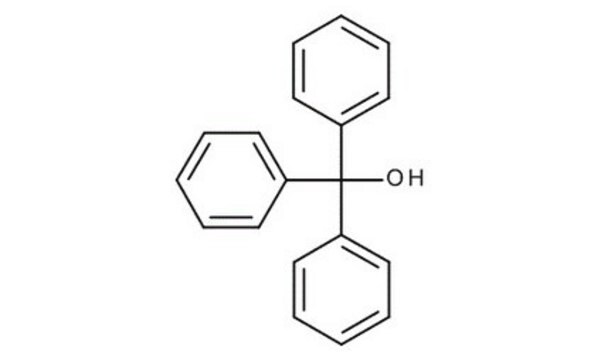
Triphenylmethanol
97%
Synonym(s):
Triphenylcarbinol, Trityl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C6H5)3COH
CAS Number:
Molecular Weight:
260.33
Beilstein:
1460837
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
360 °C (lit.)
mp
160-163 °C (lit.)
solubility
dioxane: soluble 100 mg/mL, clear, colorless to faintly yellow
functional group
hydroxyl
phenyl
SMILES string
OC(c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C19H16O/c20-19(16-10-4-1-5-11-16,17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15,20H
InChI key
LZTRCELOJRDYMQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Triphenylmethanol forms 1:1 molecular complex with triphenylphosphine oxide. It is a specific clathrate host for methanol and dimethyl sulphoxide and forms clathrate inclusion complexes. It undergoes reduction to triphenylmethane by 9, l0-dihydro-10-methylacridine in the presence of perchloric acid.
Application
Triphenylmethanol was used in the synthesis of of the two-electron reduction product of pyrylogen.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Steiner
Acta crystallographica. Section C, Crystal structure communications, 56 (Pt 8), 1033-1034 (2000-08-16)
In the crystalline 1:1 molecular complex of triphenylmethanol (TPMeOH) and triphenylphosphine oxide (TPPO), C(19)H(16)O. C(18)H(15)OP, molecular dimers are formed which are linked by O-H. O=P hydrogen bonds. The dimers are aligned by sixfold phenyl embraces to form columns. The structure
Tamer T El-Idreesy et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 9(6), 796-800 (2010-05-12)
The first synthesis of the two-electron reduction product of a pyrylogen is reported. The magnitude of the experimentally determined disproportionation constant for a pyrylogen radical cation was used to advantage in order to provide compelling evidence for formation of this
Efficient Reduction of Triphenylmethanol to Triphenylmethane by 9, l0-Dihydro-10-methylacridine in the Presence of Perchloric Acid.
Ishikawa M, et al.
Chemical Society, Analytical Methods Committee, Analyst, 62, 3754-3756 (1989)
Specific entrapment of methanol and dimethyl sulphoxide (DMSO) by a simple host compound (triphenylmethanol). Crystal structures of the Ph3COH? MeOH (1: 1) and Ph3COH? DMSO (2: 1) clathrate inclusion complexes.
Weber E, et al.
Journal of the Chemical Society. Chemical Communications, 17, 1195-1197 (1989)
Corinne Nguyen et al.
Journal of medicinal chemistry, 49(14), 4183-4195 (2006-07-11)
We report the discovery of novel uracil-based acyclic compounds as inhibitors of deoxyuridine 5'-triphosphate nucleotidohydrolase (dUTPase), an enzyme involved in nucleotide metabolism that has been identified as a promising target for the development of antimalarial drugs. Compounds were assayed against
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 134848-250G | 4061838730336 |
| 134848-50G | 4061838730343 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service