128570
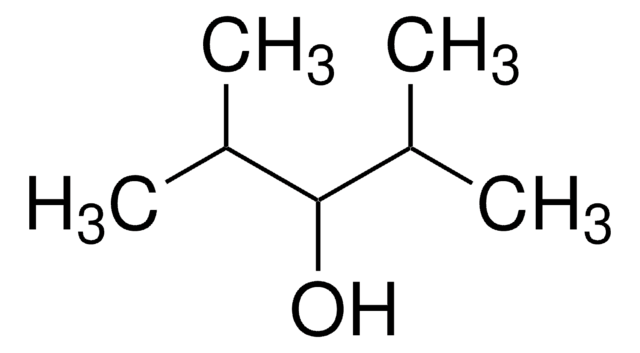
2-Hexanol
99%
Synonym(s):
(±)-2-Hexanol, Butyl methyl carbinol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
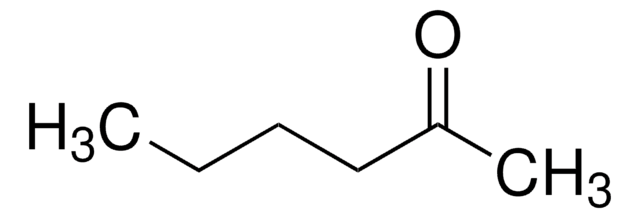
Linear Formula:
CH3(CH2)3CH(OH)CH3
CAS Number:
Molecular Weight:
102.17
Beilstein:
1718996
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.414 (lit.)
bp
136 °C (lit.)
density
0.814 g/mL at 20 °C
0.81 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
CCCCC(C)O
InChI
1S/C6H14O/c1-3-4-5-6(2)7/h6-7H,3-5H2,1-2H3
InChI key
QNVRIHYSUZMSGM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Hexanol is one of the constituent of Porella arboris-vitae extracts which has been determined by solid phase microextraction, gas chromatography-mass spectrometry (SPME GC-MS).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
114.8 °F - closed cup
Flash Point(C)
46 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Amit Kumar Tyagi et al.
Evidence-based complementary and alternative medicine : eCAM, 2013, 382927-382927 (2013-02-01)
The chemical composition of Porella arboris-vitae extracts was determined by solid phase microextraction, gas chromatography-mass spectrometry (SPME GC-MS), and 66 constituents were identified. The dominant compounds in methanol extract of P. arboris-vitae were β-caryophyllene (14.7%), α-gurjunene (10.9%), α-selinene (10.8%), β-elemene
P Manini et al.
Toxicology letters, 108(2-3), 225-231 (1999-10-08)
Since n-hexane metabolites are excreted as glucuronide conjugates, most conventional analytical procedures require preliminary hydrolysis, yielding to the 'total' 2,5-hexanedione (2,5-HD), but also giving rise to a number of artifacts. The whole pattern of n-hexane metabolites, both conjugated and unconjugated
Christina Onaca et al.
Journal of bacteriology, 189(10), 3759-3767 (2007-03-14)
Pseudomonas veronii MEK700 was isolated from a biotrickling filter cleaning 2-butanone-loaded waste air. The strain is able to grow on 2-butanone and 2-hexanol. The genes for degradation of short chain alkyl methyl ketones were identified by transposon mutagenesis using a
M Sakura et al.
Journal of comparative physiology. A, Neuroethology, sensory, neural, and behavioral physiology, 188(10), 787-797 (2002-12-06)
The capability of the cockroach Periplaneta americana to discriminate odors of structurally similar aliphatic alcohols was studied by using an operant conditioning paradigm. Cockroaches were trained to discriminate three odors: one odor associated with sucrose solution (reward) and two odors
S J Crosbie et al.
Human & experimental toxicology, 16(3), 138-145 (1997-03-01)
1. The role of skeletal muscle microsomes as a site of extrahepatic xenobiotic metabolism using n-hexane as a model substrate was investigated. The observed cytochrome P450-dependent metabolism was compared with that found with liver, and brain microsomal fractions. 2. Rat
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service