1176007
USP
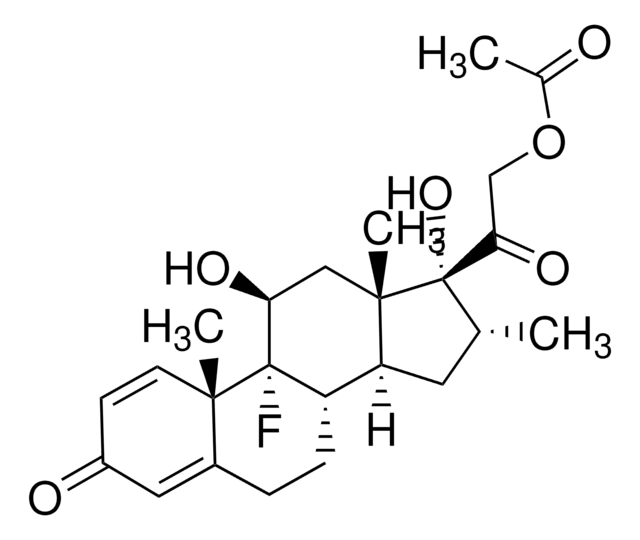
Dexamethasone
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
(11β,16α)-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione, 9α-Fluoro-16α-methyl-11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione, 9α-Fluoro-16α-methylprednisolone, Prednisolone F
About This Item
Recommended Products
biological source
synthetic
grade
pharmaceutical primary standard
Agency
USP
vapor pressure
<0.0000001 kPa ( 25 °C)
API family
dexamethasone
packaging
pkg of 125 mg
manufacturer/tradename
USP
color
white
mp
262-264 °C (lit.)
solubility
acetone: sparingly soluble
chloroform: slightly soluble
ethanol: sparingly soluble
ether: very slightly soluble
methanol: sparingly soluble
water: practically insoluble
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
SMILES string
C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(O)C(=O)CO
InChI
1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1
InChI key
UREBDLICKHMUKA-CXSFZGCWSA-N
Gene Information
human ... NR3C1(2908)
Looking for similar products? Visit Product Comparison Guide
General description
Application
Also used to prepare system suitability and standard solution during the performance test and impurity analysis by using liquid chromatography in conjunction with UV detector according to the given below monographs of United States Pharmacopeia (USP) :
- Dexamethasone.
- Dexamethasone Tablets.
- Dexamethasone Oral Solution.
- Dexamethasone Sodium Phosphate Ophthalmic Solution.
Biochem/physiol Actions
Analysis Note
Other Notes
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
A simple, precise and sensitive Reverse-Phase High Pressure Liquid Chromatography gradient method was adapted for traceability, homogeneity and total chromatographic analysis of Dexamethasone. The given experimental conditions follow the USP43-NF38 monograph method for Dexamethasone Assay and Organic Impurity Profiling. Dexamethasone, Betamethasone, Dexamethasone acetate and Desoximetasone were baseline resolved within 20 minutes using a Titan C18 UHPLC column (2.1 x 100 mm, 1.9 µm).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service