R5000
Ribonuclease A from bovine pancreas
Type II-A, ≥60% (SDS-PAGE), >= 60 Kunitz units/mg protein
Synonym(s):
Pancreatic Ribonuclease, RNAsea, RNase A, Ribonucleate 3′-pyrimidinooligonucleotidohydrolase
About This Item
Recommended Products
biological source
bovine pancreas
Quality Level
type
Type II-A
form
solid
specific activity
>= 60 Kunitz units/mg protein
mol wt
~13,700
concentration
≥60% (SDS-PAGE)
technique(s)
cell based assay: suitable
impurities
salt, essentially free
suitability
suitable for molecular biology
application(s)
diagnostic assay manufacturing
storage temp.
−20°C
SMILES string
[nH]1cnc(c1)CC(NC(=O)CCN)C(=O)O
InChI
1S/C9H14N4O3/c10-2-1-8(14)13-7(9(15)16)3-6-4-11-5-12-6/h4-5,7H,1-3,10H2,(H,11,12)(H,13,14)(H,15,16)
InChI key
CQOVPNPJLQNMDC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
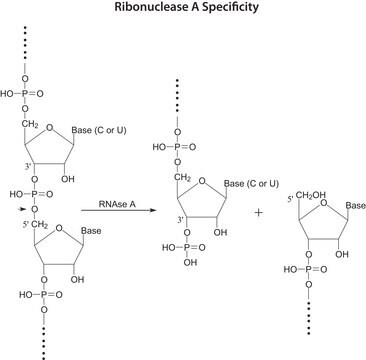
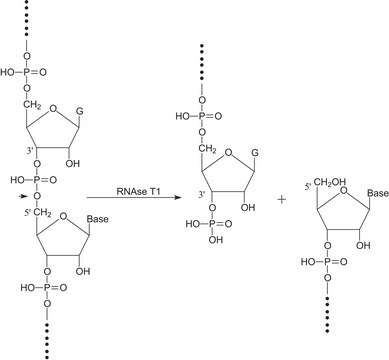
General description
Application
- RNase A is used to remove RNA from DNA plasmid and genomic DNA preparations and protein samples.
- RNase A is also used in RNA sequence analysis and protection assays.
- RNase A has been used as a tool for computer-aided drug design.
- RNase A supports the analysis of RNA sequences.
- RNase A hydrolyze RNA contained in protein samples.
- Purification of DNA is supported by RNase A.
Features and Benefits
Preparation Note
Analysis Note
Application
inhibitor
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
This procedure may be used for determination of Ribonuclease A (RNase A) activity.
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service