M1508
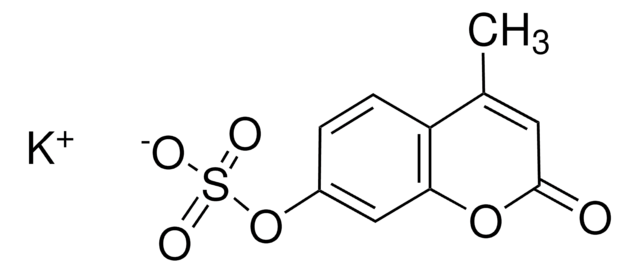
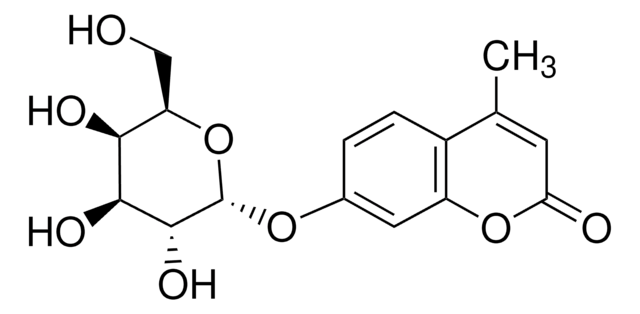
4-Methylumbelliferone sodium salt
≥98% (HPLC), crystalline
Synonym(s):
β-Methylumbelliferone, 7-Hydroxy-4-methylcoumarin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C10H7O3Na
CAS Number:
Molecular Weight:
198.15
EC Number:
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
crystalline
color
yellow
solubility
water: 50 mg/mL
SMILES string
[Na+].CC1=CC(=O)Oc2cc([O-])ccc12
InChI
1S/C10H8O3.Na/c1-6-4-10(12)13-9-5-7(11)2-3-8(6)9;/h2-5,11H,1H3;/q;+1/p-1
InChI key
JGMQHDNPUCPRQE-UHFFFAOYSA-M
General description
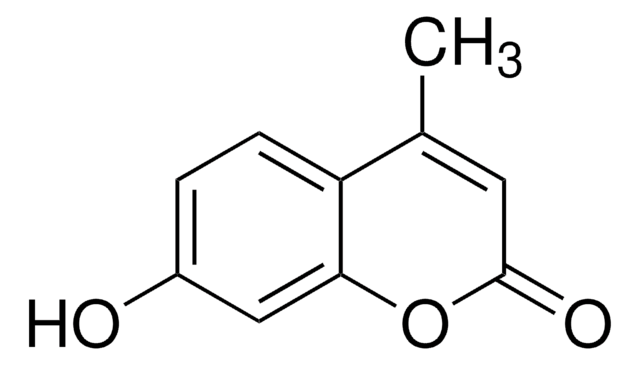
4-methylumbelliferone (4-MU) is a coumarin derivative.
Application
4-Methylumbelliferone sodium salt has been used:
- as a substrate to analyze the enzyme kinetics of its glucuronidation in the human liver
- as a standard to quantify the free 4-methylumbelliferone released as a result of enzyme-substrate action
- in cell survival assay to inhibit hyaluronan (HA) production and to study its effect on chemoresistance in ovarian cancer
Biochem/physiol Actions
4-methylumbelliferone (4-MU) serves as a hyaluronan (HA) synthesis blocker. It exhibits anti-inflammatory, anti-fibrogenic, and antitumor properties. 4-MU is used to treat biliary spasms and might also be beneficial in nonalcoholic steatohepatitis (NASH), inflammation, hepatocyte injury, and fibrotic response. 4-MU is known to be glucuronidated by most of the uridine 5ฺ-diphospho-glucuronosyltransferase (UGT) isoforms of the human liver. This makes it a potent probe substrate to examine the overall hepatic UGTs activity.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Valérie Jaulneau et al.
Journal of biomedicine & biotechnology, 2010, 525291-525291 (2010-05-07)
The industrial use of elicitors as alternative tools for disease control needs the identification of abundant sources of them. We report on an elicitor obtained from the green algae Ulva spp. A fraction containing most exclusively the sulfated polysaccharide known
Peter Pushko et al.
Virology, 501, 176-182 (2016-12-10)
Avian influenza (AI) viruses circulating in wild birds pose a serious threat to public health. Human and veterinary vaccines against AI subtypes are needed. Here we prepared triple-subtype VLPs that co-localized H5, H7 and H9 antigens derived from H5N1, H7N3
Rebecca M DuBois et al.
PLoS pathogens, 7(12), e1002398-e1002398 (2011-12-07)
Highly pathogenic avian influenza viruses of the H5N1 subtype continue to threaten agriculture and human health. Here, we use biochemistry and x-ray crystallography to reveal how amino-acid variations in the hemagglutinin (HA) protein contribute to the pathogenicity of H5N1 influenza
Ai-Sheng Xiong et al.
PloS one, 6(11), e26773-e26773 (2011-11-19)
A β-glucuronidase variant, GUS-TR3337, that was obtained by directed evolution exhibited higher thermostability than the wild-type enzyme, GUS-WT. In this study, the utility of GUS-TR337 as an improved reporter was evaluated. The corresponding gus-tr3337 and gus-wt genes were independently cloned
Gale E Smith et al.
Virology, 509, 90-97 (2017-06-19)
Avian influenza A (H5N1) viruses represent a growing threat for an influenza pandemic. The presence of widespread avian influenza virus infections further emphasizes the need for vaccine strategies for control of pre-pandemic H5N1 and other avian influenza subtypes. Influenza neuraminidase
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service